 |
 |
AbstractActinomycosis is a rare acute-to-chronic bacterial infection caused by Actinomyces. A 7-year-old healthy girl presented with left-sided nasal obstruction and purulent discharge. She had no special medical or trauma history, and the symptoms developed gradually after severe vomiting one month prior to the visit. Nasal endoscopy revealed white-black-colored cheesy material that appeared as a foreign body or fungal material in the left nasal cavity. The lesion was completely removed endoscopically under local anesthesia. The patient was prescribed only a topical antibiotic ointment without any additional systemic antibiotics. She was diagnosed with actinomycosis based on histopathological examination after a few days. There was no recurrence at six months post-procedure. The common treatment for actinomycosis is surgical removal and a systemic antibiotic therapy. However, this case was successful with complete surgical removal and only a short-term topical antibiotic ointment therapy. Therefore, the treatment for actinomycosis should differ based on clinical characteristics and the patient’s condition.
IntroductionActinomycosis is a rare human disease caused by Actinomyces. Actinomyces belong to the normal flora of the oral cavity, gastrointestinal tract, and urogenital tract. In general, due to its weak infectivity in the human body, it usually causes infection through damaged mucosa in patients undergoing long-term steroid treatment, or patients with diabetes or an immunodeficiency disease [1-5]. Although the most common clinical form of actinomycosis is cervicofacial, involvement of the nose, such as the nasal cavity, is extremely rare [1-13].
We report a rare case of nasal actinomycosis caused by residual vomiting in the nasal cavity in of a healthy child with no special medical history. The purpose of the study was explained to patient and her guardians, with all providing written informed consent for use of patient’s medical informations. The study protocol was approved by the Institutional Review Board of our hospital.
CaseA 7-year-old healthy girl presented to our hospital with one month of nasal obstruction and mucopurulent nasal discharge. The patient had no medical or dental problems and had no history of surgery or trauma. The patient’s guardian said that she had severely vomited one month prior, and at that time, part of the vomit had been regurgitated into the nasal passages and was discharged out of the body through the nostrils. The guardian recalled that, the symptoms seemed to have occurred a few days after the vomiting event. About two weeks after the onset of symptoms, she visited a local pediatric clinic, was diagnosed with a common cold, and took the prescribed medication for one week, but the symptoms persisted. She then visited our outpatients department (OPD).
Nasal endoscopy confirmed a dark black substance in the form of a mass that partially obstructed the space in the left nasal cavity (Fig. 1A). On paranasal sinus CT, a mass-like material with a clear boundary that did not invade other tissues was observed in the left nasal cavity. The lesion was round and boundary of calcified material appeared to have been formed around the lesion (Fig. 2). The lesion was removed endoscopically under local anesthesia in the OPD. No abnormal findings on the mucosa were observed after removal (Fig. 1B). Additional mucosal biopsies were not performed to check for infiltration into other tissues because of the patient’s inability to cooperate. Therefore, only the specimens that were successfully removed were biopsied. At the time of removal, the patient was considered to have a simple foreign body, and no additional systemic antibiotic treatment was administered. A mupirocin ointment was prescribed for the patient to self-apply to the lesion site for one week. Owing to the patient’s personal circumstances (quarantine due to close contact with COVID-19), follow-up was performed after two weeks. At that time, the previous biopsy results had confirmed actinomycosis (Fig. 1C). No recurrence or other specific findings were observed on nasal endoscopy. Therefore, regular follow-up was performed on an OPD basis without additional prescription of any drugs. At six months post-procedure, normal nasal mucosa was observed on nasal endoscopy without relapse or worsening.
Discussion
Actinomyces are normal flora in human mucosal tissue, such as the oral cavity and gastrointestinal and genital organs [1-14]. However, sometimes it can become pathogenic. This is called actinomycosis and usually occurs following the destruction of normal mucosal tissue in the presence of other bacteria, especially in anaerobic environments, which enables the growth of these colonies. Therefore, most cases occur after surgical procedures, such as abdominal surgery, intrauterine device insertion, or dental extractions. In addition, systemic diseases, such as diabetes and immunosuppression, are known risk factors for actinomycosis [1-5]. However, in our case, the patient had no risk factors other than severe vomiting prior to the diagnosis. Therefore, in this case, it seems that Actinomyces, which exists as a normal strain in the gastrointestinal tract, moved into the nasal cavity due to nasal reflux of gastric contents through vomiting and formed a lesion. Although it has already been found in various studies that vomit or gastric acid reflux into the nasal cavity can act as a causative agent for inflammatory diseases, such as chronic rhinosinusitis (CRS), this is the first case in which intranasal actinomycosis is assumed to be caused by vomiting [15].
Actinomycosis can be classified: cervicofacial, thoracic, abdominopelvic, central nervous system, musculoskeletal, or disseminated. Approximately 60% occur in the cervicofacial region, most of which are found in the mandible, cheek, and chin [14]. Rhinologic actinomycosis is extremely rare, and has been mostly reported in the sinuses. According to our literature review, besides our case, there have only been 12 cases reported of actinomycosis occurring in the nasal cavity, such as the nasal septum, nasal floor, or the turbinates (Table 1) [1-6,8-13]. Actinomyces is an anaerobic bacterium. Therefore, for this organism to act as a pathogen in the human body, it is necessary that the surrounding environment be anaerobic. Since the nasal cavity is continuously exposed to airflow, this anaerobic environment is not well-maintained, so the incidence is considered very rare [1-13].
Actinomycosis can also be classified into acute, subacute, or chronic, according to the rate of disease progression. In most cases, it has a chronic course that progresses to lesions, such as granulation tissue or abscess formation, but in some reports, it can lead to acute progression and even death [4-10]. Given the non-specific symptoms associated with rhinologic actinomycosis, such as nasal obstruction, foul odor, epistaxis, and mucopurulent rhinorrhea, it is difficult to differentiate it from CRS often causing a delay in diagnosis [15]. However, in this case, the patient had no prior rhinitis-related symptoms, and the lesion was removed before becoming chronic since the symptom presentation was relatively clear despite the patient’s age and treatment was provided early. For this reason, proper removal of the lesion was performed before the bacterial tissue had penetrated the mucosa, and the lesion could heal without the need to remove additional mucosa.
Unlike most bacterial infectious diseases, which are diagnosed based on positive culture results of pathogenic bacteria, diagnosing actinomycosis may be difficult as a result of the low isolation rate of bacteria. In this case, culture result were negative. Therefore, actinomycosis should be diagnosed based on clinical examinations, histopathological findings, and the presensce of sulfur granules [7,14]. If sulfur granules are observed in the exudates of the lesion, actinomycosis may be suspected. However, the probability of diagnosing sulfur granules through histopathological examination is approximately 30% [1,3,7]. While they can also be found in other diseases, such as noncardiosis, the presence of filamentous gram-positive rods and sulfur granules on histopathological examination may be considered a characteristic finding of actinomycosis. These findings were also confirmed by histopathological examination in our case. The eosinophilic material observed at the periphery of the mass was composed of filamentous or granular bacterial colonies with long, slender, straight, or wrinkled black features, which is suggestive of Actinomyces (Fig. 1C). CT scans can be helpful to determining the exact location and extent of involvement, as well as identifying any bony destruction, although there is no uniform characteristic that can absolutely confirm a diagnosis [10].
Actinomycosis treatment involves the administration of penicillin-based antibiotics and surgical removal of the lesion, but clinically, a combination of medical and surgical treatment is recommended [7,14]. However, there is no established treatment protocol for actinomycosis owing to its rarity. Surgical debridement is required to remove the source of the infection or infected tissue and drain the abscess. Long-term antibiotic therapy is recommended following surgery. Although high-dose penicillin is the drug of choice, multiple antibiotics have been used and can be tailored to the severity, location, and extension of the disease and health status of the patient. In patients with penicillin allergies, tetracycline, clindamycin and erythromycin may be used [1-6]. For this case, endoscopic surgical removal was performed at the OPD, and mupirocin topical ointment was prescribed for one week post-procedure. No additional systemic antibiotics were administered. In other words, the titer of the antibiotics prescribed to our patients was very low compared to the penicillin-based systemic antibiotic protocol that is generally prescribed for actinomycosis [4]. However, at the regular follow-ups at two weeks and six months after surgery, no recurrence was observed, and the symptoms had disappeared. Therefore, surgery and short-term topical antibiotic treatment for this patient were considered effective. This is presumably because the pathogenic potency was not strong, as the lesion was caused by accidental colonization of Actinomyces by the reflux of vomit in the absence of damage to the nasal mucosal tissue. In addition, the patient’s lesion was removed relatively early, and thus infiltration into the surrounding tissues did not progress.
In conclusion, although nasal actinomycosis is rare, it should be considered for the differential diagnosis of a mass-like lesion that looks like a foreign body on endoscope or a calcified nasal cavity mass on CT. Treatment for nasal actinomycosis has not yet been established, but in the case of a relatively mild disease, as in our case, tailored therapy may be applied that takes into account the patient’s condition and the severity of the lesion.
ACKNOWLEDGMENTSThe purpose of the study was explained to patient and her guardians, with all providing written informed consent for use of patient’s medical informations. The study protocol was approved by the Institutional Review Board of our hospital.
NotesAuthor Contribution Conceptualization: Su Young Jung, Young Min Mun. Data curation: Young Min Mun, Sang Kwon Im. Formal analysis: Young Min Mun. Resources: Young Min Mun, Gyu Man Lee. Supervision: Su Young Jung. Writing—original draft: Young Min Mun, Su Young Jung. Writing—review & editing: Su Young Jung. Fig. 1.Nasal endoscopic and histopathologic findings. A: Endoscopic findings of the left nasal cavity revealed a yellow-whitish material adhering to the left nasal septum and a dark brown-colored mass adjacent to the inferior turbinate. B: Relatively normal nasal mucosal findings after endoscopic removal of the mass lesion. C: The eosinophilic material seen at the periphery of the mass is composed of filamentous or granular bacterial colonies without fungal spores. The filamentous structures are showing long, slender, straight or wrinkled black features suggestive of Actinomyces. 
Fig. 2.Preoperative paranasal sinus CT scan findings. Coronal (A) and axial (B) views of the left nasal cavity showing a round calcified peripheral mass (arrow) in the left nasal cavity. No bone destruction or invasion was observed. 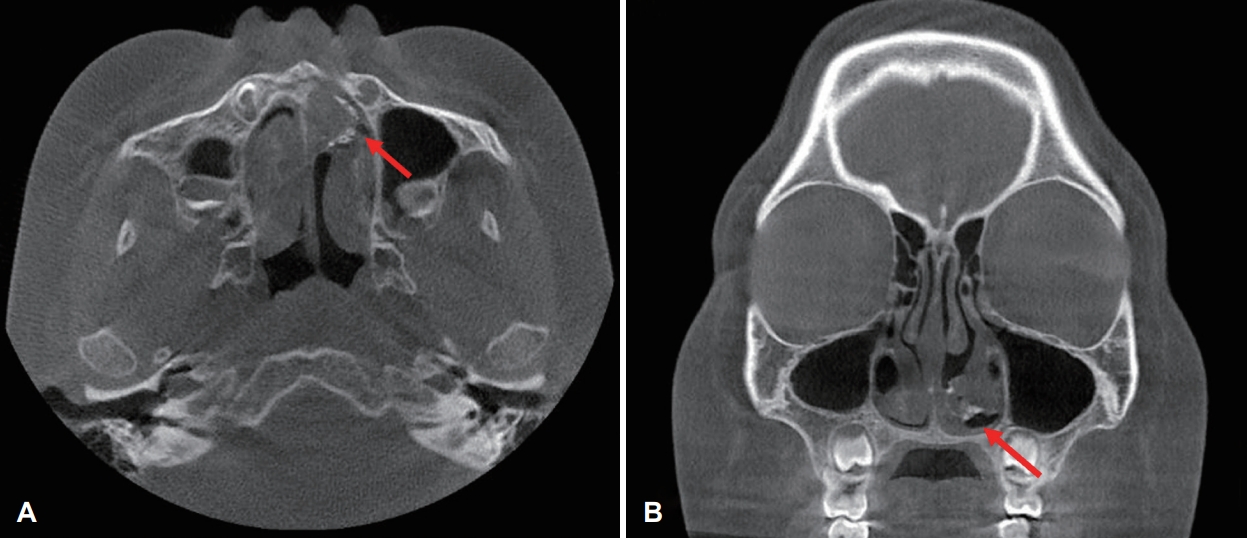
Table 1.Literature review of nasal cavity actinomycosis
REFERENCES1. Lombo C, Matos C, Fernandes F. Actinomycosis of the nasal septum: A rare entity. Cureus 2021;13(11):e19475.
2. Kingdom TT, Tami TA. Actinomycosis of the nasal septum in a patient i nfected with the hu man im mu nodef iciency vi r us. Otolaryngol Head Neck Surg 1994;111(1):130-3.
3. Lee JH, Jeong JY, Kim JS, Hoe SJ. A case of chronic noninvasive actinomycosis in the nasal cavity. Korean J Otorhinolaryngol-Head Neck Surg 2017;60(3):144-7.
4. Sakuma Y, Yamashita Y, Shiono O, Oridate N. Actinomycosis arising from the nasal cavity, with rare fatal progression. BMJ Case Rep 2016;2016:bcr2015213747.
5. Ozcan C, Talas D, Görür K, Aydin O, Yildiz A. Actinomycosis of the middle turbinate: An unusual cause of nasal obstruction. Eur Arch Otorhinolaryngol 2005;262(5):412-5.
6. Park KS, Lee DH, Lim SC. Actinomycosis of the nasal cavity. Braz J Otorhinolaryngol In press 2021.
7. Valour F, Sénéchal A, Dupieux C, Karsenty J, Lustig S, Breton P, et al. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist 2014;7:183-97.
8. Kamogashira T, Matsumoto N, Numakura S, Kikuchi Y, Ito K. Rhinolithiasis caused by actinomyces with a foreign body. IDCases 2020;19:e00718.
9. Lee DH, Yoon TM, Lee JK, Lim SC. Nasal septum actinomycosis mimicking mucocele. J Craniofac Surg 2020;31(2):e147-9.
10. Kim SD, Kim DS, Choi KU, Cho KS. Nasal cavity actinomycosis mimicking rhinolith. J Craniofac Surg 2018;29(3):e255-7.
11. Kim JS, Noh SJ, Ryu SH. Osteoma with actinomycosis in a nasal cavity: A case report. Medicine (Baltimore) 2017;96(51):e9376.
12. Batzakakis D, Karkos PD, Papouliakos S, Leong SC, Bardanis I. Nasal actinomycosis mimicking a foreign body. Ear Nose Throat J 2013;92(7):E14-6.
13. Zalagh M, Akhaddar A, Benariba F. Chronic rhinorrhea revealing an actinomycotic rhinolithiasis with ectopic tooth. Int J Oral Maxillofac Surg 2012;41(3):297-9.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |