구인두암에서 인간 유두종 바이러스 유전자 검사와 p16 과발현의 임상적 의의
Clinical Significance of Human Papillomavirus DNA Test and p16 Overexpression in Oropharyngeal Cancer
Article information
Trans Abstract
Background and Objectives
Oropharyngeal squamous cell carcinoma (OPSCC) can be caused by human papilloma virus (HPV) infection or other factors like smoking. The 8th Edition of the AJCC Cancer Staging Manual recommends different staging and treatment approaches based on etiology. Despite criticisms of its low specificity, the current guidelines suggest using p16 immunohistochemistry (IHC) as a surrogate marker for the HPV-related OPSCC. This study assessed the reliability of p16 as a surrogate marker by correlating the survival rates of OPSCC patients with the results of p16 IHC and HPV-DNA testing.
Subjects and Method
A retrospective analysis was performed on patients treated for tonsil squamous cell carcinoma at a tertiary medical institution between 1994 and 2018. All patients underwent p16 immunostaining and HPV-DNA chip tests. Out of 88 patients, 17 were excluded due to insufficient data or secondary primary cancer, leaving 71 patients.
Results
Among the 71 patients, 51 were p16 positive and 49 were HPV-DNA positive; both tests were associated with extended survival. However, discrepancies were noted in 18 patients: specifically, 11 patients were p16 positive but HPV-DNA negative, displaying a different survival pattern compared to HPV-associated and non-HPV-associated patients.
Conclusion
Both p16 immunostaining and HPV-DNA testing have their pros and cons. p16 immunostaining is cost-effective but has lower specificity. The study found discrepancies in 18 patients, suggesting that relying solely on p16 immunostaining may have limitations. It would be advisable to complement it with additional tests like the HPV-DNA chip test to predict the disease’s prognosis more accurately.
Introduction
According to global cancer statistics 2018, there were 92887 new cases of oropharyngeal cancer, resulting in 51005 deaths [1]. The most common type of cancer that develops in the oropharynx is squamous cell carcinoma, which accounts for approximately 90% [2]. Generally, two different etiologies are involved in the development of oropharyngeal squamous cell carcinoma (OPSCC). OPSCC may be related to human papilloma virus (HPV) infection, smoking, and alcohol consumption. While the incidence of HPV-negative cancers is decreasing due to reduced smoking and alcohol consumption, the overall incidence of OPSCC is on the rise because of the increasing prevalence of HPV infection [3-5]. Since HPV-positive OPSCC has significant genetic, epidemiologic and clinical differences compared to HPV-negative OPSCC, finding out the cause of carcinoma in OPSCC is important in determining treatment plans and predicting the prognosis of a patient [6-13].
In HPV-positive OPSCC, the HPV E6 and E7 proteins deactivate pRB, thereby interrupting the downstream p16ink4a-CDK4-pRB pathway. This leads to an accumulation of the cyclin-dependent kinase inhibitor p16ink4a within the cell and cell nucleus [14]. Therefore, the 8th Edition TNM classification recommends detection of overexpressed p16 by immunohistochemistry (IHC) as a surrogate marker of HPV infection. In addition, TNM staging will vary according to the p16 detection result [15]. However, using IHC to identify p16 overexpression has its limitations, including low specificity and the possibility of false positives due to perturbations in other cellular signaling pathways [16].
The aim of this study is to evaluate the concordance between the existence of HPV-DNA and overexpression of p16 in OPSCC tumors, and to examine the prognostic implications of p16 and HPV-DNA statuses for OPSCC patients.
Subjects and Methods
Patients
This study involved 88 patients diagnosed with tonsil squamous cell carcinoma, who received treatment at a tertiary medical institution between the years 1994 and 2018. Inclusion criteria were based on the availability of relevant clinical and pathological data.
Seven-teen patients were excluded due to reasons such as insufficient medical records, presence of a secondary primary cancer, or distant metastasis at diagnosis. As a result, the study included a total of 71 patients. The mean duration for tracking patient outcomes was 47.9 months, ranging from 6 to 191 months. Assessments were conducted based on the American Joint Committee on Cancer’s staging guidelines [17]. Clinical factors were reviewed retrospectively by patient chart review. Treatment modality was determined through a multidisciplinary consultation consisting of otolaryngologists, oncologists, radiation oncologists, pathologists and radiologists. Local recurrence was ascertained through clinical and imaging evaluations, and subsequently confirmed via biopsy.
This retrospective study received approval from the Institutional Review Board (approved decision number 2018AN0338).
Immunohistochemical staining and HPV-DNA chip test
A total of 85 tissue samples underwent IHC staining for p16 staining and polymerase chain reaction (PCR) test for HPV-DNA detection. The samples from primary tumors were fixed in paraffin, and the samples were sliced to a thickness of 5 micrometers, and then placed on slides, after which the paraffin was removed. Each slide was subjected to standard staining with hematoxylin and eosin (H&E). For IHC, we applied a monoclonal mouse anti-human p16 antibody (MTM Laboratories, Heidelberg, Germany). For negative controls, instead of the primary antibody, non-immune serum immunoglobulin G and phosphate-buffered saline were used. Overexpression of p16 was determined when cells showed staining in a block pattern. If the staining was focal or generally weak and diffuse, it was considered negative. A pathologist analyzed the immunostaining in at least 10 high power fields under a microscope magnified 200 times.
Before conducting the HPV-DNA chip test, sections stained with H&E were examined to verify the presence of sufficient tumor tissue. The paraffin was then removed, and DNA was isolated from the tissue using a QIAamp DNA mini kit (Qiagen, Valencia, CA, USA). The HPV-DNA chip test for HPV genotyping was performed using a PCR based on a DNA microarray system. The HPV-DNA Chip used in this study can detect 32 types of HPV, distinguishing between 19 high-risk types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 69, 70, 73) and 13 low-risk types (6, 11, 32, 34, 40, 42, 43, 44, 54, 55, 62, 81, 83).
Statistical analysis
Statistical tests were conducted to examine the relationship between survival rates, various clinical factors, HPV-DNA positivity, and p16 overexpression. The relationship between HPV and p16 status was assessed using Pearson’s chi-square test with Yates’ continuity correction. Overall survival (OS) and disease free survival (DFS) curves were plotted using the KaplanM-eier method, and differences were assessed with the Log-rank test. Independent variables underwent univariate analysis first. Significantly related variables were then further analyzed using the Cox proportional risk regression model in a multivariable analysis. In this study, a p-value of less than 0.05 was considered statistically significant. Statistical analyses were carried out using SPSS software version 22.0 (IBM Corp., Armonk, NY, USA).
Results
OS rate according to clinical characteristics
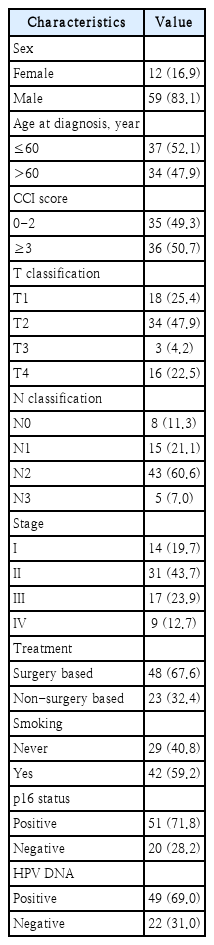
The clinical characteristics of the 71 patients are detailed in Table 1. Patients had a median age of 61.2 years at diagnosis, with the majority (59 patients or 83.1%) being male. The average duration of follow-up was 47.9 months. The Charlson Comorbidity Index was calculated for each patient, resulting in an average score of 3 and ranging between 0 and 7. Of the patients, 48 underwent surgical treatments, which in some cases were combined with postoperative RT or CCRT. The remaining 23 patients were treated with either RT or CCRT alone.
For p16 immunostaining, 51 patients tested positive while 20 tested negative. Out of 71 patients, HPV-DNA was detected in 49 (69.0%). Among these, HPV-16 was the predominant type, found in 37 of the 49 infected tumors, accounting for 75.5%. HPV-58 was identified in 4 tumors. HPV-18 was present in 2 tumors. Regarding low-risk HPV genotypes, HPV-6 was detected in 2 tumors. 2 tumors had a combination of HPV-81, HPV-6, and HPV-11. Another 2 tumors exhibited both HPV-11 and HPV-40.
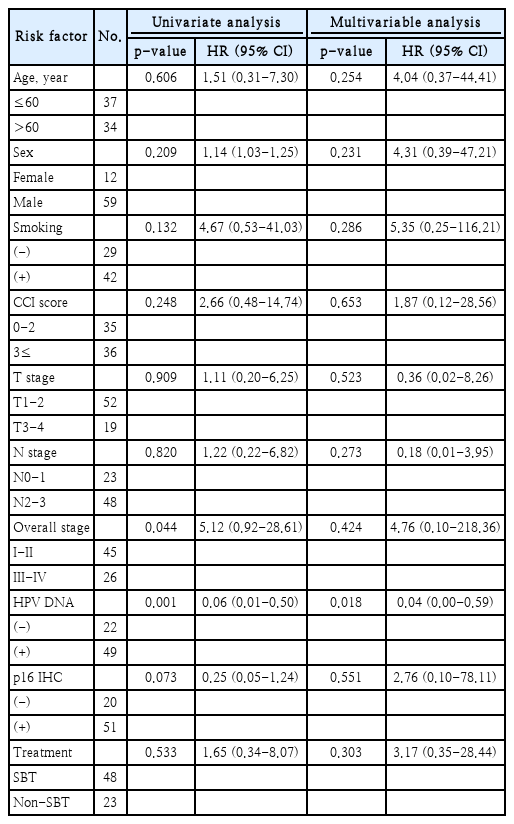
Univariate analysis revealed that overall stage and the results of the HPV-DNA chip test as significant prognostic indicators for OS. Other assessed factors did not demonstrate significant prognostic value (Table 2).
Overlap and discrepancy between p16 overexpression and HPV-DNA status
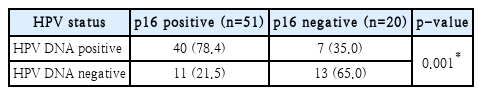
In 51 patients with OPSCC, p16 overexpression was observed. Despite confirming p16 overexpression in these patients, HPV-DNA was not detected in 11 of them, accounting for 21.5%. Additionally, in 20 patients where p16 overexpression was not observed, HPV-DNA was detected in 7, representing 35.0%. Consequently, the similarity in the results of the two test methods was statistically significant (p<0.05). However, there were noticeable discrepancies observed (Table 3).
Prognostic significance of p16 overexpression
The mean OS in months for p16-positive OPSCC patients was 178.02, while for p16-negative patients it was 68.46. Similarly, the mean DFS in months for p16-positive patients was 137.38, compared to 52.20 for p16-negative patients. The presence of p16 in primary tumors was associated with significantly longer OS (p=0.023) and DFS (p=0.046) in OPSCC patients (Fig. 1).
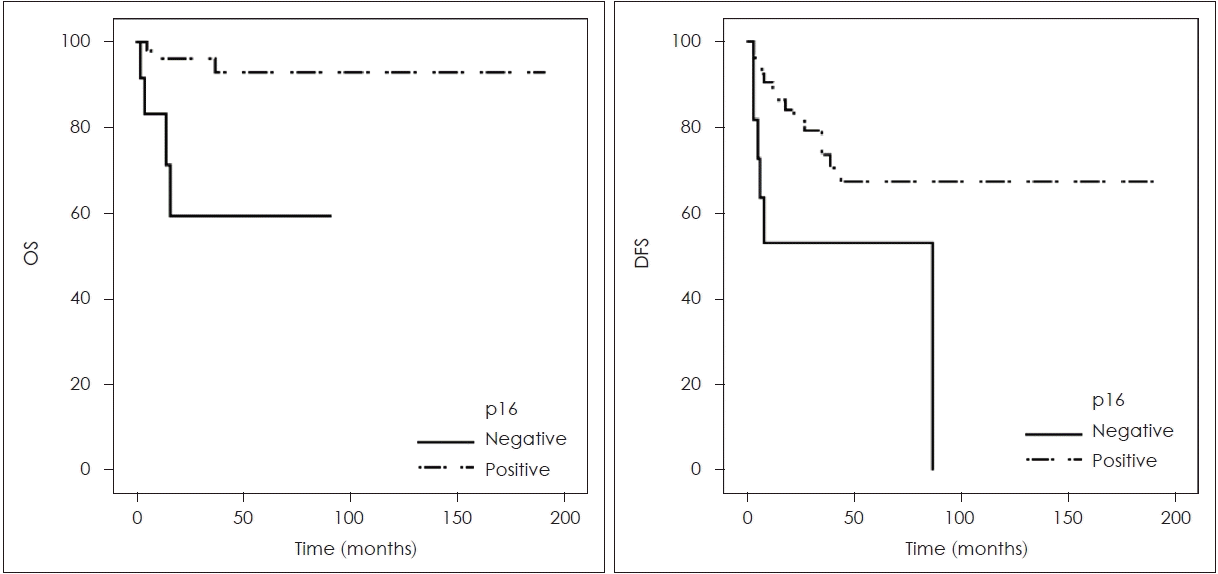
Overall survival (OS) rate and disease free survival (DFS) rate according to p16 immunohistochemistry results.
The average OS for HPV-DNA-positive OPSCC patients was 186.77 months, compared to 93.51 months for those who were HPV-DNA-negative. The presence of HPV-DNA in primary tumors was associated with an extended OS rate (p=0.001). Additionally, the mean DFS for those testing positive with the HPV-DNA chip was 135.37 months, while it was 70.96 months for those testing negative. Although there was a trend suggesting a difference in DFS between the two groups, this was not statistically significant (p=0.095) (Fig. 2).
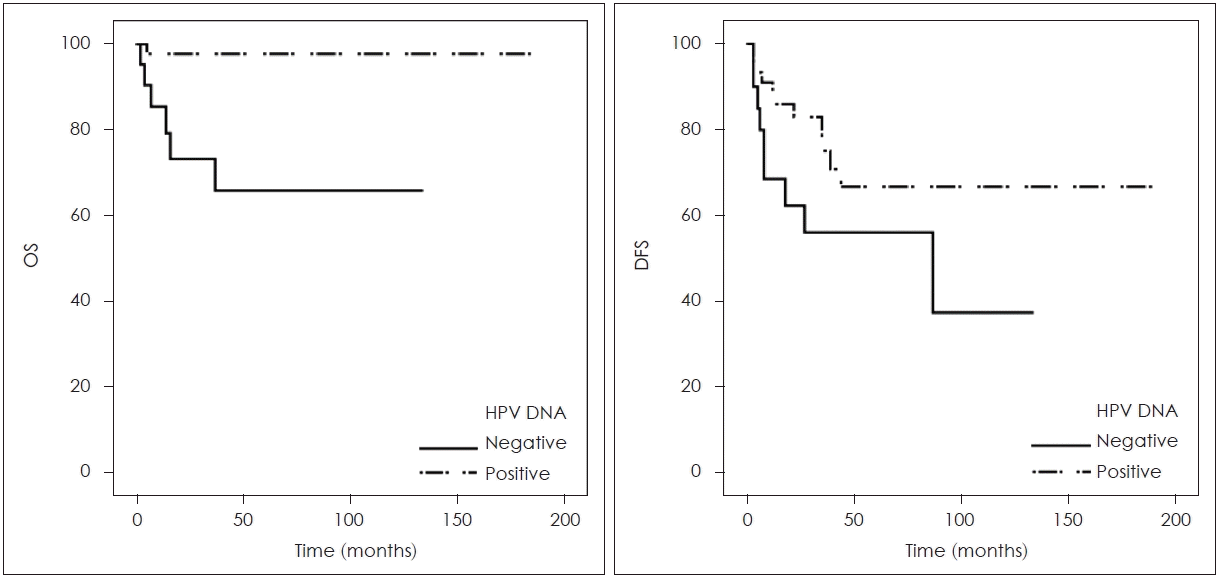
Overall survival (OS) rate and disease free survival (DFS) rate according to human papilloma virus (HPV)-DNA chip test results.
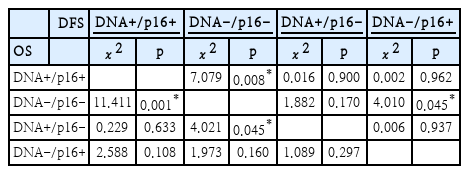
When considering the results from both the p16 IHC and HPV-DNA chip tests, patients who tested positive for both HPV-DNA and p16 (HPV-DNA(+)/p16 IHC(+) group) exhibited a significant difference in OS compared to those negative for both tests, the HPV-DNA(-)/p16 IHC(-) group (p=0.001). In cases where there were discrepancies between the HPV-DNA and p16 test outcomes, a significant survival rate difference was observed only in the subset of patients positive for HPV-DNA but negative for p16 IHC (HPV-DNA(+)/p16 IHC(-) group), when compared to the HPV-DNA(-)/p16 IHC(-) group (p=0.045). Conversely, no such difference was observed in the HPV-DNA(-)/p16 IHC(+) group (Fig. 3 and Table 3).
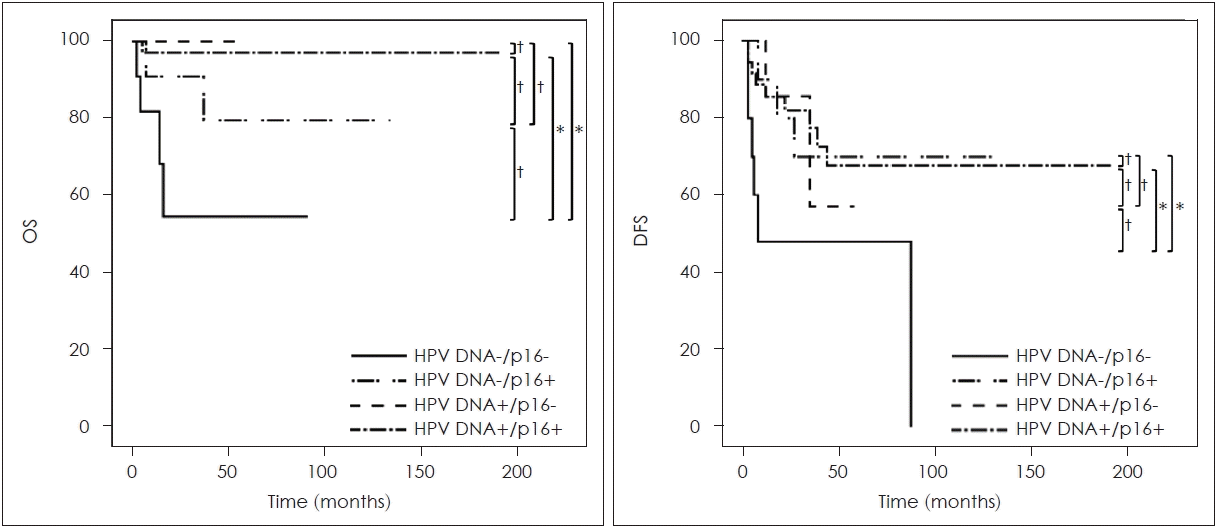
Overall survival (OS) rate and disease free survival (DFS) rate according to p16 immunostaining and human papilloma virus (HPV)-DNA chip test results. *p<0.05 with Log-rank test; †p>0.05 with Log-rank test.
In the comparison of DFS, analogous to the OS analysis, the HPV-DNA(+)/p16 IHC(+) group demonstrated a markedly better survival outcome than the HPV-DNA(-)/p16 IHC(-) group (p=0.008). However, the DFS outcomes differed between the two discrepant groups: there was no significant difference between the HPV-DNA(-)/p16 IHC(-) group and the HPV-DNA(+)/p16 IHC(-) group (p=0.170). In contrast, a significant difference was observed when comparing the HPV-DNA(-)/p16 IHC(-) group to the HPV-DNA(-)/p16 IHC(+) group (p=0.045) (Fig. 3 and Table 4).
Multivariable analyses of prognostic variables
In the multivariable Cox regression assay, only HPV-DNA chip test results showed a significant correlation with the prolonged OS (HR=0.04, 95% CI=0.00-0.59, p=0.018) (Table 2).
Discussion
In OSPCC, lesions induced by HPV are known to constitute 50%-60% of all cases. Furthermore, this proportion is increasing due to a rise in HPV infections [3-5,8,18,19]. OSPCC associated with HPV typically has a favorable prognosis, a finding that aligns with the results of this study. However, discrepancies have emerged in infection test results when using two different diagnostic tools: p16 IHC and the HPV-DNA chip test. Moreover, variations in prognosis were observed based on the discrepancies between these two test outcomes.
Presently, the predominant diagnostic tools employed for the detection of HPV-DNA are principally based on PCR. PCR possesses the advantage of high specificity and sensitivity. However, it necessitates the utilization of costly analytical equipment and the deployment of trained personnel. Furthermore, this assay can solely authenticate the presence of HPVDNA within the tissue. Consequently, it is untenable to ascertain whether the HPV-DNA is situated within the tumor or the adjacent normal tissue, nor is it feasible to validate whether the HPV-DNA participated in the tumor’s formation. Conversely, the detection of p16 overexpression by IHC has the advantage of being low-cost and employing a simple test method, making it accessible globally [20]. Currently, it is recommended to assign cancer staging for HPV-related OPSCC when there is a nuclear staining pattern on p16 IHC, a staining intensity of 2+ or 3+, and 75% or more of the cancer cells are stained [17].
However, Robinson, et al. [16] and Westra [21] have highlighted a limitation to the detection of p16 overexpression by IHC due to its low specificity and the possibility of false positives arising from perturbations in other cellular signaling pathways. The lack of internationally uniform standards for determining test results is also problematic.
In this study, it was observed that the overexpression of p16 can manifest in patients in whom HPV-DNA is undetected, and, conversely, instances exist where HPV-DNA is identifiable but no overexpression of p16 is evident. Distinct differences in prognosis were discernable compared to those patients who tested either negative or positive in both assessments. Although the study did not achieve consistent statistical significance in predicting OS or DFS, the findings imply the potential of encountering false positives in p16 assessments and false negatives in HPV-DNA chip tests. Furthermore, the study suggests the plausible occurrence of p16 overexpression due to alterations in other cellular signaling pathways in the absence of an HPV infection, denoting potential limitations in prognostic predictions premised on p16 expression.
Numerous preceding studies have substantiated that the overexpression of p16 can serve as a surrogate marker for HPV-related OPSCC, and this study also found a correlation between the results of p16 IHC and improved OS and DFS. Thus, due to the favorable prognosis of p16 IHC-positive OPSCC patients, attempts are being made to apply de-intensified treatment [22]. However, as there are instances of poor prognosis in some HPV-related OPSCC patients, caution is warranted in utilizing p16 as a surrogate marker. This necessity for caution is believed to be associated with the discrepancies between p16 IHC and HPV-DNA test results observed in this study. Therefore, it is advisable to conduct the HPVDNA chip test along with p16 IHC rather than using only the p16 IHC to determine if the oropharyngeal cancer is caused by HPV. However, as mentioned earlier, expensive equipment like the microarray scanner for the DNA chip test is required. This presents a challenge for developing countries or small medical facility that may not have such facilities, making it difficult to conduct the test [20]. Hence, additional research is imperative to elucidate the reasons for the differing results between p16 IHC and HPV-DNA tests, so that a better surrogate marker that compensates for the limitations of the two tests can be proposed.
Previous research has argued that when there is a difference in the results of the two tests, particularly when there is overexpression of p16 but the HPV-DNA test is negative, HPV chromogenic in situ hybridization (CISH) should be conducted to verify the presence or absence of HPV-DNA in the tumor. If it is revealed to be negative in the consensus HPV CISH, it has been suggested that it should be considered a cancer type not related to HPV [16,21].
The limitations of this study lie in the fact that the overall number of subject patients was not large, and additionally, the number of patients showing discrepancy in the two tests was small. Therefore, there is a possibility that bias may have occurred in the survival rate analysis. Thus, subsequent studies involving a larger number of patients are deemed necessary.
In conclusion, according to the results of this study, when both p16 IHC and HPV-DNA chip tests are accessible, conducting both tests concurrently may enhance the precision in prognosticating the patient’s outcome and facilitate the selection of a more suitable treatment strategy. Given that this study inherits the generic limitations of retrospective chart review studies and constraints due to the restricted number of patients included, further investigations involving larger cohorts are imperative.
Acknowledgements
This study was supported by the Clinical Trial Center of Korea University Anam Hospital (I1502411) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF2018R1D1A1A09083263, 2021R1F1A1056645,2023R1A2C1004538).
Notes
Author Contribution
Conceptualization: Seung-Kuk Baek. Data curation: Jaehyeong Kim. Formal analysis: Jaehyeong Kim, Juhyun Lee. Funding acquisition: Seung-Kuk Baek. Investigation: Jaehyeong Kim. Methodology: Jaehyeong Kim, Seung-Kuk Baek. Project administration: Seung-Kuk Baek. Supervision: Kwang Yoon Jung, Soon-Young Kwon, Jeong-Soo Woo, Jae-Gu Cho. Validation: Kyoung-Ho Oh. Writing—original draft: Jaehyeong Kim, Seung-Kuk Baek. Writing—review & editing: Juhyun Lee.