자폐스펙트럼장애가 있는 인공와우 환자의 어음 인지 및 매핑 특성: 10년 사용 후 비교 결과
Speech Perception and Mapping Characteristics of Cochlear Implant Patients With Autism Spectrum Disorder: Comparative Results After 10 Years of Use
Article information
Trans Abstract
Background and Objectives
This study aimed to analyze postoperative performance and mapping characteristics of cochlear implants (CIs) by comparing patients with autism spectrum disorder (ASD) to those without ASD, and to suggest CI mapping solutions in patients with ASD.
Subjects and Method
This retrospective study enrolled 10 children with ASD and hearing disabilities, who received simultaneous bilateral CI (ASD group), and 20 children with bilateral hearing disabilities, who received simultaneous bilateral CI at the same age (control group). CI performance was analyzed using speech perception tests (categorical auditory performance score and monosyllable, bisyllable, and Ling’s 6 tests) and a sound field test. The mapping characteristics focused on variables related to stimulus intensity and fine-tuning.
Results
The performance of the ASD group was significantly poorer than that of the control group in all speech perception and sound field tests. At the comfortable (C) and threshold (T) levels, the ASD group scored significantly lower than the control group. The dynamic range of ASD group was significantly narrower than the control group. The ASD group had significantly lower pulse width, sensitivity, and volume than control group.
Conclusion
CI mapping in the ASD group showed practical limitations. To avoid overstimulation in patients with ASD, the dynamic range should be set narrow, or the C/T level should be set lower than normal. Key control factors, such as pulse width, sensitivity, and volume, should be set lower than the control group. Although lower performance from CI is generally expected in the ASD group, CI mapping in the ASD group requires a long-term approach with dedicated efforts and patience.
Introduction
Cochlear implant (CI) placement is an effective hearing rehabilitation surgery for correcting severe hearing loss that cannot be corrected with a hearing aid [1]. As most pre-deaf babies are detected early by the national newborn hearing screening, CI is emerging as a promising surgical treatment for hearing rehabilitation [2,3]. Surgical technology and mechanical advances in CIs have advanced remarkably. Advances in devices and technology have increased the success rate of CI and postoperative hearing results, particularly for congenital hearing loss [4,5].
CI mapping refers to the customized programming of CIs to cater to the specific needs of its user, enabling them to hear optimal sound from the electrodes of CIs array [6]. The process involves various factors, including setting the thresholds (T)- and comfortable (C)- levels, as well as adjusting various parameters, such as pulse width (control of stimulation rate), sensitivity (fine adjustment of sound processor), and volume [6]. In practice, postoperative rehabilitation, including mapping and speech therapy, is essential to obtain maximum outcome after CI surgery [6].
Autism spectrum disorder (ASD) is a relatively common disorder in individuals with congenital hearing loss (1/150) [7,8]. It is comorbid with congenital hearing loss and severe hearing loss in 1.7%-5.3% and 3.5% of patients, respectively [7,8]. According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the main characteristic of ASD is persistent deficits in social communication and social interaction, which include problems with social initiation, nonverbal communication, and social awareness and insight [9-11]. Problems with social initiation and response means deficits in social-emotional reciprocity, ranging from abnormal social approach and failure of normal back and forth conversation (through reduced sharing of interests, emotions, and affect and response) to total lack of initiation of social interaction. Problems with nonverbal communication include deficits in nonverbal communicative behaviors used for social interaction, ranging from poorly integrated verbal and nonverbal communication (through abnormalities in eye contact and body language or deficits in understanding and using nonverbal communication) to a total lack of facial expressions or gestures. Problems with social awareness and insight include deficits in developing and maintaining relationships according to their developmental level (beyond those with caregivers), ranging from difficulty in adjusting behavior to suit different social contexts (through difficulty in sharing imaginative play and making friends) to an apparent lack of interest in people. In particular, a lack of “theory of mind” and the inability to take another person’s perspective is concerning [9-11]. Another characteristic of ASD is restricted, repetitive patterns of behavior, interests, or activities (atypical speech, rituals, preoccupations, atypical sensory behaviors) [9-11]. Early signs of ASD include delayed speech development, spin object, hyperactivity, preference to play alone, rejecting cuddles, and sleep problems. Compared to infants with ASD, healthy infants tend to maintain good eye contact and social skills, sit relatively still, reach developmental milestones, and respond more adaptively in high-sensory areas [12].
As cochlear implantation is performed early after birth, the presence of ASD is may not be immediately recognized. It is discovered after undergoing CI surgery and attempting mapping for hearing rehabilitation. CI mapping and rehabilitation are extremely difficult owing to the uncooperative and unresponsive nature of children with ASD. Rehabilitation and evaluation of outcomes in ASD [13] are associated with many challenges. Even when treated in the medical field, children with ASD do not make eye contact or perform repetitive movements and cannot sit still. They are particularly hypersensitive to loud sounds or flashing lights and tend to reject CIs. However, only a few studies have examined the mapping characteristics of CI patients with ASD. The present study aimed to report the mapping characteristics and outcomes of CI by comparing patients with ASD to patients without ASD. Furthermore, we considered mapping solutions for CI infants with ASD based on their experiences at a tertiary hospital.
Subjects and Methods
Screening protocol
In this retrospective study, we analyzed the mapping data of children with simultaneously placed bilateral CIs between January 2019 and January 2020. Additionally, we analyzed data of 10 children with ASD who had received simultaneous bilateral CIs and had been using them for approximately 10 years. Moreover, a comparative analysis was performed for 20 children without ASD who received simultaneous bilateral CIs during the same period. The inclusion criteria were as follows: 1) prelingual hearing loss, 2) CI mapping and auditory rehabilitation at a tertiary hospital CI center, 3) simultaneous bilateral ‘CochlearTM Nucleus® contour advance’ (Cochlear, New South Wales, Australia) CI user, 4) usage of CI for more than 8 h a day, 5) no inner ear anomalies, and 6) no central nervous system disease.
The CI performance was analyzed using categorical auditory performance (CAP); Ling’s 6 sound test; speech perception tests including the monosyllable, bisyllable, and Ling’s 6 tests; and CI-aided sound field test. The mapping characteristics focused on variables related to stimulus intensity (C level, T level, dynamic range, input dynamic range, and volume) and those related to fine-tuning (pulse width, sensitivity, frequency allocation, and stimulation rate). This study was approved by the Institutional Review Board of the tertiary hospital (KUMC IRB AN0509).
Patients
Table 1 shows the demographics of patients without ASD and with ASD using simultaneous bilateral CIs. For the analysis, 20 children without ASD (control group) were compared with 10 children with ASD. Among the children with ASD, male exhibited dominance over female, with a ratio of approximately 4:1. The age for children without ASD and those with ASD was 10.5±2.6 years and 11.7±1.6 years, respectively. The age of first cochlear implantation for children without ASD and those with ASD was 2.1±0.8 years and 2.0±0.5 years, respectively. The average duration of CI use for children without ASD and those with ASD was 8.4±2.1 years and 9.7±1.6 years, respectively. No significant differences were observed in age, mean age at first CI, or period of CI use between the control and ASD groups. All the patients underwent regular CI mapping and auditory rehabilitation.
ASD was diagnosed by pediatric neurologist, using the DSM-5 criteria. All three symptoms of persistent deficits in social communication and social interaction had to be present, with at least two of the four autistic symptoms related to restricted, repetitive patterns of behavior, interests, or activities (atypical speech, rituals, preoccupations, atypical sensory behaviors). Among the 10 children diagnosed with ASD, 5 of them were at Level 2 (“requiring substantial support”) and 5 were at Level 3 (“requiring very substantial support”) according to the DSM-5 severity classification.
Statistical analysis
All values were presented as mean±standard deviation using SPSS 13.0 statistical program (SPSS Inc., Chicago, IL, USA). The unpaired t test was used for comparative data, and p<0.05 was considered to be statistically significant (***p< 0.001, **p<0.01, and *p<0.05).
Results
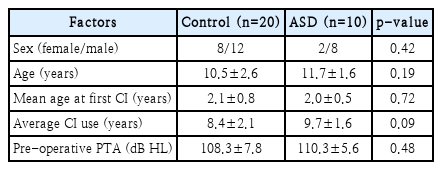
Fig. 1 presents the CAP scores. According to the CAP, children without ASD and those with ASD achieved scores of 6.6±0.5 and 2.9±0.6, respectively. According to the monosyllabic test, children without ASD and those with ASD obtained a result of 78.5%±10.1% (range: 60-90) and 14.0%±13.5% (range: 0-40), respectively. According to the bisyllabic test, children without ASD and those with ASD obtained a result of 85%±7.1% (range: 70-95) and 17.5%±17.5% (range: 0-50), respectively. According to Ling’s 6 test, children without ASD and those with ASD obtained a result of 93.3%±8.5% (range: 75-100) and 28.5%±15.6% (range: 10-50), respectively (Fig. 2). The ASD group demonstrated poorer performance (p<0.001) than the control group in all speech perception tests.
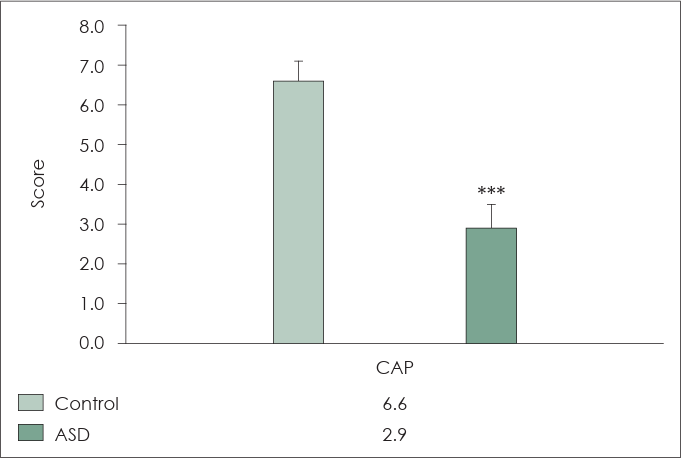
Results of categorical auditory performance (CAP) following regular cochlear implant mapping and auditory rehabilitation. Children without autism spectrum disorder (ASD) exhibit a score of 6.6±0.5, whereas children with ASD exhibit a score of 2.9±0.6. The ASD group exhibit poorer performance than that of the control group (***p<0.001).
Results of speech perception test following regular cochlear implant mapping and auditory rehabilitation. According to the monosyllable, bisyllable, and Ling’s 6 tests, the autism spectrum disorder (ASD) group demonstrates poorer performance (***p<0.001) than that of the control group in all speech perception tests. In all speech perception tests, the performance of children with ASD is significantly poorer than that of children without ASD.
After regular CI mapping and auditory rehabilitation, a sound field test, also known as CI-aided pure-tone audiometry (PTA), was performed. CI-implanted children without ASD could hear at 34.3±3.4 dB HL, whereas CI-implanted children with ASD could only hear at 53.0±6.3 dB HL. In the sound field test, the hearing threshold of the ASD group was higher/worse (p<0.001) than that of the control group in the sound field test (Fig. 3).

Following regular cochlear implant (CI) mapping and auditory rehabilitation, sound field test (CI-aided pure tone audiometry) was performed. CI-implanted children without autism spectrum disorder (ASD) could hear at 34.3±3.4 dB HL, whereas CI-implanted children with ASD could only hear at 53±6.3 dB HL. The hearing threshold of the ASD group was higher/worse (***p<0.001) than that of the control group in the sound field test.
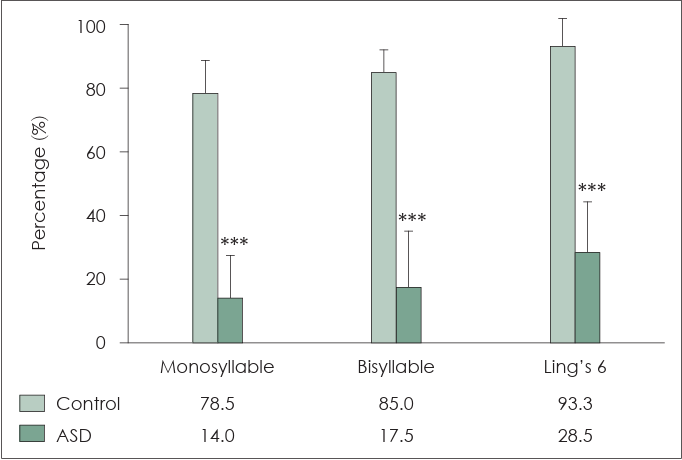
Fig. 4 compares the characteristics related to CI mapping. The C level of children without ASD and those with ASD was 195.1±10.0 CL and 165.8±11.2 CL, respectively. The T level for children without ASD and those with ASD was 138.4±13.8 CL and 118.0±12.5 CL, respectively. The dynamic range (DR) for children without ASD and those with ASD was 57.1±9.5 CL and 47.8±7.1 CL, respectively. The input DR level for children without ASD and those with ASD was 43.0±7.7 CL and 42.0±2.6 CL, respectively. The ASD group exhibited lower C (p<0.001) and T (p<0.01) levels, as well as narrower DR (p<0.05) than the control group. The input DR was not significantly different between the two groups (p=0.69). In children with ASD, lowering of C/T levels and narrowing of the DR were observed to avoid overstimulation. Nevertheless, reducing the input DR received by the CI speech processor did not appear to be necessary.
Comparison of characteristics related to cochlear implant (CI) mapping. At the comfortable (C) level, the performance of children without autism spectrum disorder (ASD) is 195.1±10 CL and that of children with ASD is 165.8±11.2 CL. At the threshold (T) level, the performance of children without ASD is 138.4±13.8 CL and that of children with ASD is 118±12.5 CL. At the dynamic range (DR), the performance of children without ASD is 57.1±9.5 CL and that of children with ASD is 47.8±7.1 CL. At the input DR level, the performance of children without ASD is 43±7.7 CL and that of children with ASD is 42±2.6 CL. The ASD group has lower C (***p< 0.001) and T (**p<0.01) levels and narrower DR (*p<0.05) than those of the control group. The input DR was not significantly different (p=0.69). In children with ASD, lowering C and T levels and narrowing DR help to avoid over-stimulation. However, reducing the input DR received by the CI speech processor is not necessary.
Fig. 5 depicts a comparison of the CI mapping parameters. The pulse width (25-300 μs) was set to 32.9±7.2 μs and 25±0 μs for children without ASD and those with ASD, respectively. The sensitivity (levels 1-20; default 12) was set to level 14.4±0.7 and 10.3±0.7 for children without ASD and those with ASD, respectively. The volume (levels 1-9) was set to level 6.5±0.7 and 4.3±0.5 children without ASD and those with ASD, respectively. The ASD group exhibited lower pulse width (p<0.001), sensitivity (p<0.001), and volume (p<0.001) than control group.
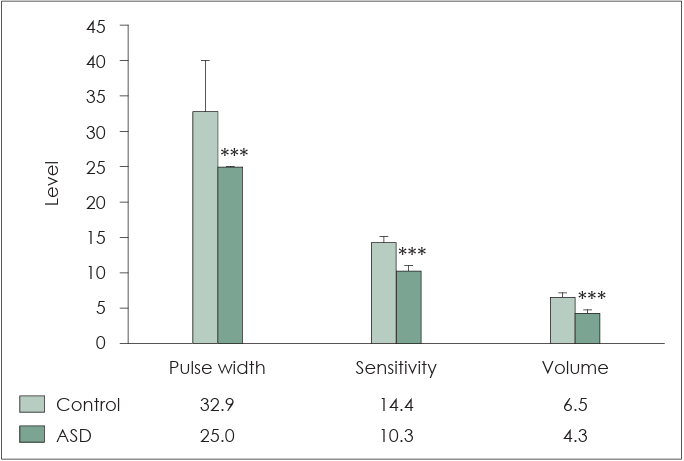
Comparison of cochlear implant mapping parameters. The pulse width (25–300 µs) was set to 32.9±7.2 µs for children without autism spectrum disorder (ASD) and 25±0 µs for children with ASD. The sensitivity (levels 1–20; default 12) was set to level 14.4±0.7 for children without ASD and 10.3±0.7 for children with ASD. The volume (levels 1–9) was set to level 6.5±0.7 for children without ASD and 4.3±0.5 for children with ASD. The ASD group has lower pulse width (***p<0.001), sensitivity (***p<0.001), and volume (***p<0.001) than those of the control group.
In addition, Fig. 6 depicts a nonsignificant comparison of the CI mapping parameters. In the frequency allocation (default: 7938), the children without ASD and those with ASD exhibited a frequency allocation of 7763±335.4 Hz and 7938±0 Hz, respectively. In stimulation rate (default: 900), children without ASD and those with ASD exhibited a rate of 930±92.3 per second and 900±0 per second. No significant differences were observed in frequency allocation (p=0.11) or stimulation rate (p=0.32) in either group. We attempted to improve speech perception in children without ASD by slightly increasing the stimulation rate. In contrast, in children with ASD, increasing the stimulation rate or changing the frequency assignment was difficult because of their hyper-responsiveness.
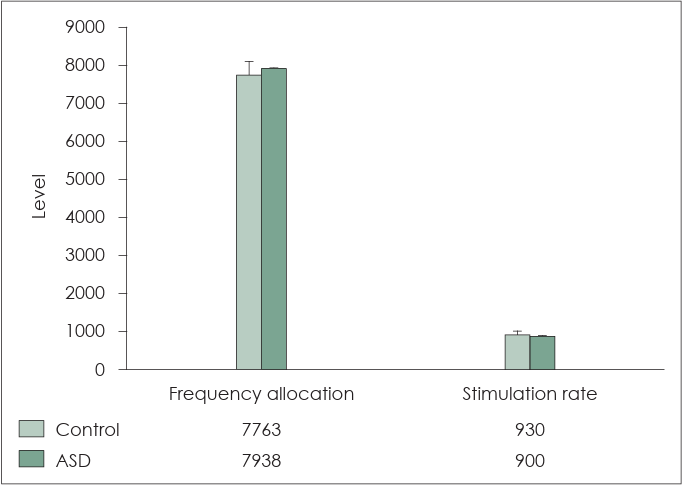
Comparison of cochlear implant mapping parameters. In frequency allocation (default: 7938), children without autism spectrum disorder (ASD) exhibit a frequency allocation of 7763±335.4 Hz, whereas children with ASD exhibit 7938±0 Hz. The stimulation rate (default: 900) is 930±92.3 per s in children without ASD and 900±0 per second in children with ASD. No significant differences are observed in frequency allocation (p=0.11) and rate (p=0.32) in both the groups. Attempts have been made to improve speech perception by slightly narrowing the frequency allocation in children without ASD. An attempt was made to improve speech perception by slightly increasing the stimulation rate in these children. In contrast, in children with ASD increasing the stimulation rate or changing the frequency assignment was challenging owing to their hyper-responsiveness.
Discussion
The present study demonstrated that children with ASD who received CI experienced significantly poor results in speech recognition test, despite proper CI operation timing, adequate wearing/training period, CI mapping and rehabilitation. In addition, to avoid excessive stimulation, CI medical staffs were compelled to set a narrow DR, while setting the C/T level lower than that of children without ASD. Key control factors, such as pulse width, sensitivity, and volume should also be set lower than those in children without ASD to avoid excessive stimulation. In particular, increasing C/T levels rapid or indiscriminately should be avoided because children with ASD have difficulty in perceiving sound effectively. CI mapping in children with ASD is a gradual and lengthy process that requires patience. Thus, only 20% of children with ASD do not perform well enough to achieve control hearing rehabilitation results.
Another challenge in clinical setting was the difficulty in performing PTA and speech recognition tests on children with ASD. Therefore, even if the sound field test results were worse than normal, it did not mean that children with ASD had poor peripheral hearing. These results were presumed to be due to the unique characteristics of the children with ASD, such as the refusal to undergo hearing tests or CI training due to hypersensitivity and aversion to hearing experience.
In other studies, CI outcomes in patients with ASD were reported to be poor, and the CAP score demonstrated considerable variability [14]. Unfortunately, 45.5% of patients with ASD demonstrated no improvement over time, and only 22% of the children achieved CAP scores of 5-7. The CAP score was 1-2 in most children with ASD (72.7%), and only 18.2% of them achieved the highest level of language skills [14]. The children with ASD in the present study demonstrated an average of 35% lower electrical CI charge than that of the children with ASD reported in the literature [14]. Moreover, the variables related to CI mapping were reduced by 15%-35%.
Compared to children without ASD, children with ASD often exhibit delayed developmental milestones across various domains such as language, intelligence, emotion, social behavior, and manual/locomotor activity, even with the use of CIs [15]. Therefore, a relatively low performance is expected from children with ASD using CIs [14,16-19]. Successful mapping of CIs for children with ASD requires a long duration of dedicated effort and patience [20]. Establishing a rapport between parents and patients is essential, and a long-term involvement of familiar CI mapper and speech therapist is recommended [20,21]. Furthermore, since multiple disabilities are common in children with ASD, sophisticated and personalized CI mapping is essential [14,18,19]. In a realistic medical environment, the following problems can be summarized for CI patients with autism: 1) refusal to wear CI, 2) hypersensitivity and rejection of sound stimulation, 3) refusal of mapping and speech therapy, 4) difficulty of communication and relationship, 5) difficulty in performing hearing tests.
Notably, children with ASD are unique, and their responses to CI may vary based on specific mapping characteristics and individual needs. Therefore, assessing individual needs of each patient and addressing CI mapping factors are essential to optimize CI outcomes in children with ASD. What should be avoided in CI children with autism is to recklessly raise the C/T level or to rapidly increase the stimulus parameters of CI mapping because auditory rehabilitation development is slow.
A limitation of this study was the challenging recruitment process owing to the small number of children with ASD. Additionally, the CI training process and speech perception tests were particularly difficult for medical staff and parents because of the complex and time consuming nature of these training and mapping processes for children with ASD, emphasizing the need for social attention and national support in the future. The cause of deafness in children receiving CIs is mainly unknown, but it is thought that some hereditary deafness may be involved, and for example, studies have been published showing that myocyte enhancer factor 2 c mutation is associated with autism and hearing loss [22]. In the future, when genetic analysis is conducted on a wide range of CI recipients, genetic relationships will be revealed.
For children with ASD using CI, the C/T level should initially be low and the dynamic range should be narrow to avoid overstimulation. Key control factors, such as pulse width, sensitivity, and volume should also be set to a low level to avoid excessive stimulation. Relatively poor performance is generally expected in children with ASD using CIs. A long period of dedicated effort and patience is required to achieve the desired mapping results in children with ASD using CIs.
Supplementary Video
The Supplement is available with this article at https://doi.org/10.3342/kjorl-hns.2024.00423.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : RS-2022-KH129293). This work was supported by Korea University Research Fund (K2306671, K2310471, K2306031, K2208471, K2205081, K2125741, K2211761). These funding sources provided only financial support and played no specific scientific role in this study.
The contents of this paper were presented in Korean at the medical conference of the Korean Audiological Society on March 19, 2022, and the related video file is provided to the Korean Journal of Otorhinolaryngology-Head and Neck Surgery (Supplementary Video).
Notes
Author contributions
Conceptualization: Gi Jung Im, Euyhyun Park, Pyung Kon Tark. Methodology: Gi Jung Im, Jiwon Chang. Validation: Gi Jung Im, Euyhyun Park, Jiwon Chang. Formal analysis: Gi Jung Im. Data curation: Gi Jung Im. Visualization: Gi Jung Im. Project administration: Gi Jung Im. Funding acquisition: Gi Jung Im. Writing—original draft: Gi Jung Im, Jiwon Chang. Writing—review & editing: all authors.