 |
 |
AbstractBackground and ObjectivesThe pathogenesis of idiopathic sudden sensorineural hearing loss (ISSNHL), an otologic emergency disease, remains unclear. Several studies have attempted to illustrate the association between cytokines and ISSNHL. The purpose of this study was to evaluate the prognostic significance of various cytokines in patients with ISSNHL.
Subjects and MethodIn this case-control study, a total of 55 patients with ISSNHL underwent treatment with oral prednisolone for 2 weeks. Serum cytokine levels, including interleukins (ILs) (IL-1a, IL-1b, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12), and tumor necrosis factors (TNF-╬▒ and TNF-╬▓), were measured using human cytokine panels at first visit. Patient characteristics such as age, gender, the status of hypertension, diabetes mellitus, vertigo, time from onset to visit, and initial hearing levels were also evaluated.
ResultsSerum levels of cytokines were correlated with the prognosis of ISSNHL patients. IL-4 Ōēź0.225 (pg/mL) and TNF-╬▒ Ōēź5.155 (pg/mL) were significantly associated with poor therapeutic outcomes (OR=44.317, p=0.015 and OR=269.465, p=0.006, respectively). In addition, age and initial hearing levels were also significant prognostic factors.
IntroductionIdiopathic sudden sensorineural hearing loss (ISSNHL) is one of the most important emergency diseases in otolaryngology, defined as a sensorineural hearing loss which occurs within 3 days [1-3]. The definite cause of ISSNHL is unknown in most cases; it is usually idiopathic. Although many mechanisms, including infectious diseases, immune-mediated mechanisms, vascular occlusion, and injuries in the inner ear structures, are suggested as etiologies of the disease, their roles have not been clarified yet [2,4,5]. The reported incidence of ISSNHL varies between 3.9-160 per 100000 individuals every year [6-8]; the recovery rate ranges between 45%-65% in patients without treatment, and between 49%-79% in those with treatment [1,8,9].
Tumor necrosis factor-alpha (TNF-╬▒) and interleukins (ILs) are cytokines that play major role in immune reactions and inflammatory processes; several studies on TNF-╬▒ and ILs have reportedly illustrated the etiology of ISSNHL. Although their mechanisms are still unclear, TNF-╬▒ production and ILs are known to regulate apoptosis of the inner ear cells, which affect microcirculation of the inner ear and contribute to the inflammatory response of the cochlea [10-12]. Previous studies demonstrated that serum cytokine levels, such as IL-6, IL-8, and TNF-╬▒, significantly increased in patients with ISSNHL compared to controls [13,14]. In addition, many studies are underway that have suggested the potential use of serum cytokine levels, such as of IL-2, IL-6, IL-8, IL-10, IL-12, and TNF-╬▒, to determine the prognosis of SSNHL patients [11,15].
However, previous studies failed to demonstrate the prognostic significance of specific serum cytokine levels. Therefore, the present study aimed to analyze serum cytokine levels in patients with SSNHL, and to determine their prognostic significance. Additionally, we attempted to present the significant cutoff values for cytokine levels in order to enable proper treatment in patients with poor prognosis.
Subjects and MethodsPatientsThe present study included 55 patients (31 males, 24 females) who visited our institution between March 2014 and March 2017. All patients underwent routine physical examination including otoscopic examination, pure tone audiometry, impedance audiometry, temporal bone CT, and posterior fossa MRI with gadolinium. Patients with hearing loss of Ōēź30 dB across at least three contiguous frequencies occurring within Ōēż3 day were included. The exclusion criteria were 1) middle ear disease, 2) retrocochlear pathology, 3) poor general condition, not amenable to steroid treatment, 4) family history of deafness, 5) bilateral hearing loss, 6) treatment at another hospital, 7) history of previous SSHNL, 8) conductive or mixed hearing loss, 9) initial hospital visit occurring 10 days after hearing loss, and 10) other otologic illness such as MeniereŌĆÖs disease, perilymphatic fistula, and autoimmune inner ear disease.
Audiometric evaluationAll patients underwent a pure tone examination at their first visit. Averages of air conduction thresholds at frequencies of 500, 1000, 2000, and 4000 Hz were calculated. We classified the patients into four classes using the initial average thresholds as suggested by Demirhan, et al. [15]: mild (30-50 dB), moderate (51-70 dB), severe (71-90 dB), and profound (Ōēź90 dB) [3].
Hearing improvementAll patients were admitted to our hospital and treated with oral prednisolone for 14 days (1 mg/kg/day for 5 days, 0.8 mg/ kg/day for 3 days, 0.5 mg/kg/day for 3 days, 0.2 mg/kg/day for 3 days). Pure tone audiometry was performed 2 weeks after the end of the treatment, and the outcome of patients were divided into 4 groups as ŌĆścomplete recovery,ŌĆÖ ŌĆśpartial recovery,ŌĆÖ ŌĆśslight recovery,ŌĆÖ and ŌĆśno improvementŌĆÖ according to SiegelŌĆÖs criteria of hearing improvement [16].
Cytokine measurementBlood samples were obtained from all patients before the initiation of treatment. Serum samples were derived from the blood samples by centrifugation and were stored in a freezer at -80┬░C. Milliplex┬« MAP Human Cytokine Panel (HCYTOMAG- 60K; Millipore, Billerica, MA, USA), a stable and precise tool for detection of biomarkers, was used to measure serum levels (pg/mL) of 11 different cytokines, including IL-1a, IL-1b, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12, TNF-╬▒, and TNF-╬▓, according to the manufacturerŌĆÖs instructions [17]. In brief, 96-well plates were pre-wet with 200 ╬╝L of wash buffer for 10 min at room temperature (20┬░C-25┬░C). Wash buffer was decanted from all wells by turning over the plates and tapping onto absorbent towels. Standards or serum samples (25 ╬╝L) were added to appropriate wells, followed by addition of assay beads. Plates were incubated with agitation on a plate shaker overnight at 4┬░C or for 2 h at room temperature. The contents of wells were then removed and detection antibodies were added to each well. Plates were incubated for 1 h at room temperature. The fluorescent conjugate (Streptavidin-Phycoerythrin) was added to each well and plates were incubated for 30 min at room temperature. Fluid was then removed by vacuum and wells were washed twice. Each sample analysis was performed in duplicate. Data were collected and analyzed using Luminex 200TM (Luminex Corp., Austin, TX, USA), High Throughput Screening (HTS) System (Luminex Corp), FLEXMAP 3D┬« (Luminex Corp) and MAGPIX┬« with xPONENT┬« software (Luminex Corp).
EthicsThis study was approved by the Institutional Review Board (IRB) of our institution (Korea University Medical Center IRB: KUGH16327), and informed consent from all patients was obtained before investigation.
Statistical analysisWe used the t-test and linear-by-linear association test to analyze the difference between groups. We also used the Mann-Whitney U test to compare the cytokine levels between the recovered and unrecovered groups. In order to analyze the effect of cytokine levels on hearing recovery, we obtained the area under receiver operating characteristic (ROC) and used multivariate logistic regression. The best cut-off point was determined among the proposed points using Youden index. At the determined cut-off point, sensitivity and specificity were calculated and their range was estimated with 95% confidence interval. Statistical analysis was performed using the SPSS Software ver. 22 (IBM Corp., Armonk, NY, USA), and p<0.05 was considered statistically significant.
ResultsThis study included 55 patients (31 males and 24 females). The mean age of all the patients was 51.0┬▒15.9 year and the time from disease onset to the initial visit was 3.6┬▒2.8 day. Of all the patients, 15 patients (27.3%) experienced vertiginous dizziness, 15 patients (27.3%) had hypertension, and 9 patients (16.4%) had diabetes. When the patients were categorized according to initial hearing levels, 20 (36.4%) patients had mild hearing loss, 11 (20.0%) had moderate hearing loss, 15 (27.3%) had severe hearing loss, and 9 (16.4%) had profound hearing loss. When the patients were categorized according to the degree of hearing recovery based on SiegelŌĆÖs criteria, 20 (36.4%) patients achieved complete recovery, 9 (16.4%) achieved partial recovery, 6 (10.9%) achieved slight recovery, and 20 (36.4%) showed no improvement (Table 1).
In Table 2, the ŌĆśrecovered groupŌĆÖ consists of patients who achieved ŌĆścomplete recovery,ŌĆÖ ŌĆśpartial recovery,ŌĆÖ and ŌĆśslight recovery,ŌĆÖ while the ŌĆśunrecovered groupŌĆÖ consists of patients who showed ŌĆśno improvement.ŌĆÖ The characteristics of both groups were compared. The mean age of the patients in the unrecovered group was significantly higher than that of the recovered group patients (t-test, p=0.002), and patients with underlying hypertension were more likely to be in the unrecovered group (t-test, p=0.026). Also, there was a tendency for worse initial hearing levels in the unrecovered group, as compared to the recovered group (linear-by-linear association, p=0.005). However, there were no statistical differences between the two groups according to sex, diabetes, dizziness, and the time from disease onset to the initial visit. Based on these results, age, hypertension, and initial hearing levels were associated with the degree of hearing recovery.
Table 3 shows the comparison data of serum cytokine levels between the two groups. Since the cytokine levels do not follow a normal distribution, data was presented with the median, first quartile, and third quartile. When the two groups were compared using the Mann-Whitney U test, the IL-4 and TNF-╬▒ levels of the unrecovered group were significantly higher than those of the recovered group (p=0.005 and p=0.031, respectively). Although there were no significant differences in the other cytokines, IL-1a, IL-6, IL-10, and TNF-╬▓ were higher in the unrecovered group. Interestingly, there was no significant difference in IL-4 and TNF-╬▒ among the groups according to initial hearing level (one-way analysis of variance, p=0.484, p=0.645).
To identify the specific levels of IL-4 and TNF-╬▒ that could affect patient prognosis, the ROC curves based on IL-4 and TNF-╬▒ levels of all patients were calculated. By analyzing this, we tried to find the optimal cutoff value considering both sensitivity and specificity. The area under curve (AUC) of IL-4 was 0.727 (pg/mL) (p=0.005) (Fig. 1A) and the AUC of TNF-╬▒ was 0.676 (pg/mL) (p=0.031). When the cutoff value of IL-4 was set to 0.225 (pg/mL), the sum of sensitivity (88%) and specificity (66%) was the highest. Similarly, when the cutoff value TNF-╬▒ was set to 5.155 (pg/mL), the sum of sensitivity (70%) and specificity (71%) was the highest. The patients were categorized into two groups based on the cutoff values derived from the ROC curve analysis. Among the 28 patients with IL-4 Ōēź0.225 (pg/mL), 16 (57.1%) patients were in the unrecovered group, while among the 27 patients with IL-4 <0.225 (pg/mL), 4 (14.8%) patients were in the unrecovered group. Therefore, the patients with IL-4 Ōēź0.225 (pg/mL) showed significantly more unrecovered cases (p=0.002) (Fig. 2A). Likewise, the patients with TNF-╬▒ Ōēź5.155 (pg/mL) showed significantly more unrecovered cases (p=0.004) (Fig. 2B).
When univariate regression analysis was conducted on the unrecovered group with the previously mentioned variables, there were associations between age, hypertension, initial hearing, IL-4 Ōēź0.225 (pg/mL), TNF-╬▒ Ōēź5.155 (pg/mL), and poor hearing recovery (Table 4). However, other associations including diabetes, dizziness, and time from onset to the initial visit were not significant. Multivariate logistic regression was conducted with adjustment by the factors that showed association in the univariate regression analysis, age, hypertension, initial hearing, IL-4 Ōēź0.225 (pg/mL), TNF-╬▒ Ōēź5.155 (pg/mL), and poor hearing recovery. We also included duration from onset, since the p-value was marginally significant in univariate analysis. The odds ratio (OR) was 44.317 (p=0.015) for IL-4 Ōēź0.225 (pg/mL), and the OR was 269.465 (p=0.006) for TNF-╬▒ Ōēź5.155 (pg/mL). Furthermore, the OR with increasing age by 1 was 1.137 (p=0.029), the OR of profound initial hearing compared to mild initial hearing was 1431.676 (p=0.027), and the OR of duration by days from the onset of hearing loss was 2.228 (p=0.020) (Table 4). Therefore, we concluded that patients with older age, poor initial hearing, late onset of treatment, IL-4 Ōēź0.225 (pg/mL), and TNF-╬▒ Ōēź5.155 (pg/mL) are more likely to be classified into the ŌĆśunrecovered groupŌĆÖ after treatment. Although only partially significant in our statistical analysis, hypertension and duration from onset are also considerable risk factors of poor recovery.
DiscussionThe purpose of this study was to analyze the levels of various cytokines in patients with ISSNHL, and to identify cytokines that could predict hearing recovery in patients. Based on such study, patients who are expected to have poor prognosis could be screened before treatment, and be treated more aggressively. In this study, the levels of many cytokines were analyzed; the ORs of poor hearing recovery were found to increase with increased levels of IL-4 and TNF-╬▒.
Although there have been many recent studies conducted to analyze the mechanism of ISSNHL, the role of cytokines in the onset of ISSNHL remains unclear. It is known that cytokines are produced by various types of cells, and they regulate the proliferation and differentiation of the target cell [3,18]. Based on previous studies, Ren, et al. [14] reported that SSNHL patients had higher serum TNF-╬▒ levels than controls. Demirhan, et al. [15] reported that patients who did not respond to the treatment had significantly higher serum TNF-╬▒ levels prior to treatment than patients who responded well to the treatment. Further on, Tsinaslanidou, et al. [11] compared the expression of TNF-╬▒ between treatment days 1 and 8. The TNF-╬▒ levels of patients with good treatment results significantly decreased after treatment. This study had results similar to the previous study results, wherein the serum TNF-╬▒ levels were higher in patients with poor treatment results than in patients with good treatment results.
TNF-╬▒ is a strong pro-inflammatory cytokine, and is reported to play an important role in the immune system, in the proliferation and differentiation of cells, and apoptosis [10]. Previous studies on TNF-╬▒ expression in ISSNHL patients suggest that ISSNHL is caused by either direct or indirect inflammatory damage, and that TNF-╬▒ has a crucial role in this process [11,14,15]. However, there are no studies that have been able to clearly identify the mechanism of TNF-╬▒. Adams [19] found that TNF-╬▒ and NF-kB are abundant in the principal cell of the spiral ligament, and thus disruption of cytokine balance may result in hearing impairments. Henceforth, Keithley, et al. [18] conducted an animal study that demonstrated that TNF-╬▒ injected into the inner ear increased the number of inflammatory cells, thereby resulting in hair cell destruction. However, this study did not show a direct association to hearing impairment, and it did not provide a clear explanation of the mechanism. Further studies on the mechanism of TNF-╬▒ in the onset of SSNHL are deemed necessary.
ILs, including IL-2, IL-6, IL-8, IL-10, and IL-12, are known to be associated with the pathophysiology of ISSNHL [11,13-15]. However, there have been no studies that specifically describe their mechanism. IL-6, one of the most studied ILs, tends to increase in most inflammatory states together with TNF-╬▒. Ren, et al. [14] reported that the serum levels of TNF-╬▒ and IL-6 were higher in progressive ISSNHL patients than in controls, and suggested that this may have an important role in the pathogenesis of ISSNHL. Tsinaslanidou, et al. [11] identified the expression of TNF-╬▒, IL-2, IL-6, and IL-8 in ISSNHL patients using an enzyme-linked immunosorbent assay, and confirmed that TNF-╬▒ and IL-6 are associated with treatment prognosis. In particular, they reported that treatment results are better when the expression of TNF-╬▒ decreases while the expression of IL-6 increases. In addition, IL-6, as an inflammatory cytokine, promotes an inflammatory response via the IL-6 receptor (IL6R), which activates proinflammatory cascades, resulting in the hepatic production of C-reactive protein (CRP), fibrinogen, and other reactants [3,20,21]. It has been reported that IL6R is associated with various inflammatory conditions and vascular diseases; cardiovascular disease incidence also increases when reactant levels increase (e.g., CRP, fibrinogen, and IL-6) [22,23].
Although no studies have reported association of serum IL-4 levels with the pathogenesis of ISSNHL, Nam, et al. [24] reported that IL-4 receptor polymorphism is significantly related to increased risk of ISSNHL. IL-4, which is known for regulating cell proliferation and apoptosis in a variety of cells, plays a major role in immunoglobulin E secretion, resulting in the progression of various inflammatory conditions, such as systemic lupus erythematosus, pulmonary fibrosis, and allergic airway inflammation [25,26]. In addition, many studies have demonstrated that IL-4 interacts with TNF-╬▒ and IL-6 to amplify TNF-╬▒ levels, increase the expression of IL-6 mRNA, and enhance production of IL-6 proteins [27-30]. Although the exact role of IL-4 in the pathogenesis mechanism of ISSNHL is not clarified, it is considered that the interaction of IL-4, IL-6, and TNF-╬▒ contributes by inducing inflammatory responses in the inner ear cells or by affecting the blood supply to the inner ear. Therefore, further studies on the mechanism are deemed necessary.
Meanwhile, there are many studies on factors associated with treatment results of SSNHL patients, and recently studies suggesting models for predicting prognosis have been conducted. Based on these studies, age, tinnitus, dizziness, initial hearing levels, time from disease onset to the initial treatment, and comorbidity may be associated with prognosis of SSNHL [31-33]. Although a few studies previously reported that age and recovery of ISSNHL are not associated [34,35], recent studies repeatedly confirm that age is an important factor that can predict patient prognosis. Suzuki, et al. [31] studied the effect of patientsŌĆÖ age on prognosis in 205 patients, and reported that the possibility of hearing recovery decreases by 0.07% as the age increases by 1 year. Gianoli and Li [36] showed that patients aged below 60-year-old had a tendency of better treatment results, and Zadeh, et al. [37] reported that patients aged below 40-year-old had higher hearing recovery rates. In this study, the mean age of the patients whose condition did not improve was significantly higher than of those whose condition improved. It was concluded that the possibility of no improvement was higher with increasing age. Furthermore, initial hearing was a very strong prognostic factor and many studies reported that as initial hearing worsens, prognosis also worsens [31-33,36,37]. This study also demonstrated that patients with profound hearing loss had a higher possibility of experiencing no improvement than patients with mild hearing loss. Based on these results, it is expected that patients with older age and increased hearing loss show poor prognosis. In the current study, however, dizziness and time of treatment initiation did not affect treatment outcome, unlike in other studies. The limitations of this retrospective study are the small sample size and the possible inaccuracy of the medical records.
As with earlier studies, this study showed that inflammatory cytokines (e.g., TNF-╬▒ and IL-4) may have an important role in the pathogenesis of SSNHL, but was unable to clearly define the mechanism. Interestingly, initial hearing level and IL-4, TNF-╬▒ levels did not have significant association. Multivariate analysis reveals that elevation of IL-4 and TNF-╬▒ levels are independent risk factors for no recovery after treatment. In our study, we establish substantial evidence that specific cytokine levels hold significance in predicting post-treatment prognosis, even independent of initial hearing level. In other words, if the IL-4 and TNF-╬▒ levels prior to treatment are higher than the cutoff value, the patient is very likely to have no improvement in hearing after treatment. Therefore, through consideration of significant factors (e.g., age, initial hearing, and IL-4 and TNF-╬▒ levels), caregivers are likely to identify high risk patients, and inform patients the possibility of poor prognosis and the necessity for aggressive treatment.
An earlier study presented that a combination of hyperbaric oxygen therapy and oral steroid treatment can aid recovery in patients with profound initial hearing loss [38]. There are other studies confirming the additional benefit of hyperbaric oxygen therapy [39]. Furthermore, there are studies reporting the benefits of early intratympanic steroid injection in ISSNHL patients [40]. Although hyperbaric oxygen therapy or intratympanic injections are still controversial with regard to treatment effects, the early addition of hyperbaric oxygen therapy and intratympanic steroid injection to high-risk groups is considered potentially beneficial. For this reason, further studies are required to further explore the benefits of the aforementioned treatment on high-risk patients with increased IL-4 and TNF-╬▒.
The major limitation of this study is the small number of patients. Therefore, hearing improvement was only categorized into two groups (recovered and unrecovered). It is also possible that cytokines such as IL-1a, IL-6, IL-10, and TNF-╬▓ might have shown insignificant results due to the small sample size. Also, due to the small number of patients, we could not consider subgroup analysis of some important factors. Comorbidities such as hypertension, diabetes mellitus, and obesity may alter cytokine levels, thus further studies including such considerations will clarify our findings. However, our results were generally consistent with previous studies suggesting that certain cytokines may play a major role in ISSNHL pathogenesis. The current study is exploratory, and is the first to suggest direct association between IL-4 and ISSNHL prognosis. Our findings may help to build insight about cytokine testing in sudden hearing impairment patients. Further studies are yet needed to better understand the mechanisms behind this relationship. Also, we attempted to set up initial cut-off values for IL-4 and TNF- ╬▒ for the first time in order to give practical aid to caregivers to make decisions for aggressive treatment in the clinical setting.
In conclusion, the patientŌĆÖs age, initial hearing levels, and IL-4 and TNF-╬▒ plasma levels prior to treatment are associated with hearing recovery. Based on these, it is possible to predict patients who might have poor prognosis, warn the patients with possible poor prognosis, and provide more aggressive treatments, such as hyperbaric oxygen therapy and intratympanic steroid injection.
ACKNOWLEDGMENTSThis study was supported by a Grant-in-Aid from the department foundation of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine.
NotesAuthor Contribution Conceptualization: Su-Jong Kim, June Choi, Gi Jung Im, Sung-Won Chae, Jae-Jun Song. Data curation: Jee Won Moon. Formal analysis: Angela Yun Kim, June Choi, Gi Jung Im, Sung-Won Chae, Jae-Jun Song. Investigation: Jae-Jun Song. Methodology: Su-Jong Kim, Jae-Jun Song. Project administration: Su-Jong Kim, Seok-Youl Choi, Jee Won Moon. Supervision: June Choi, Gi Jung Im, Sung-Won Chae, Jae-Jun Song. WritingŌĆöoriginal draft: Angela Yun Kim, Su-Jong Kim. WritingŌĆöreview & editing: Angela Yun Kim. Fig.┬Ā1.ROC curves for sensitivity and specificity of IL-4 (A) and TNF-╬▒ (B). Area under ROC curve expresses the model explanation power, which was 0.727 (p=0.005) in IL-4 and 0.676 (p=0.031) in TNF-╬▒, respectively. ROC, receiver operating characteristic; IL, interleukin; TNF, tumor necrosis factor. 
Fig.┬Ā2.Distribution of hearing recovery according to serum levels of IL-4 and TNF-╬▒. A: When IL-4 Ōēź0.225 (pg/mL), the probability that the patient belongs to the ŌĆśunrecovered groupŌĆÖ is significantly higher (p=0.002). B: When TNF-╬▒ Ōēź5.155 (pg/mL), the probability that the patient belongs to the ŌĆśunrecovered groupŌĆÖ is also significantly higher (p=0.004). IL, interleukin; TNF, tumor necrosis factor. 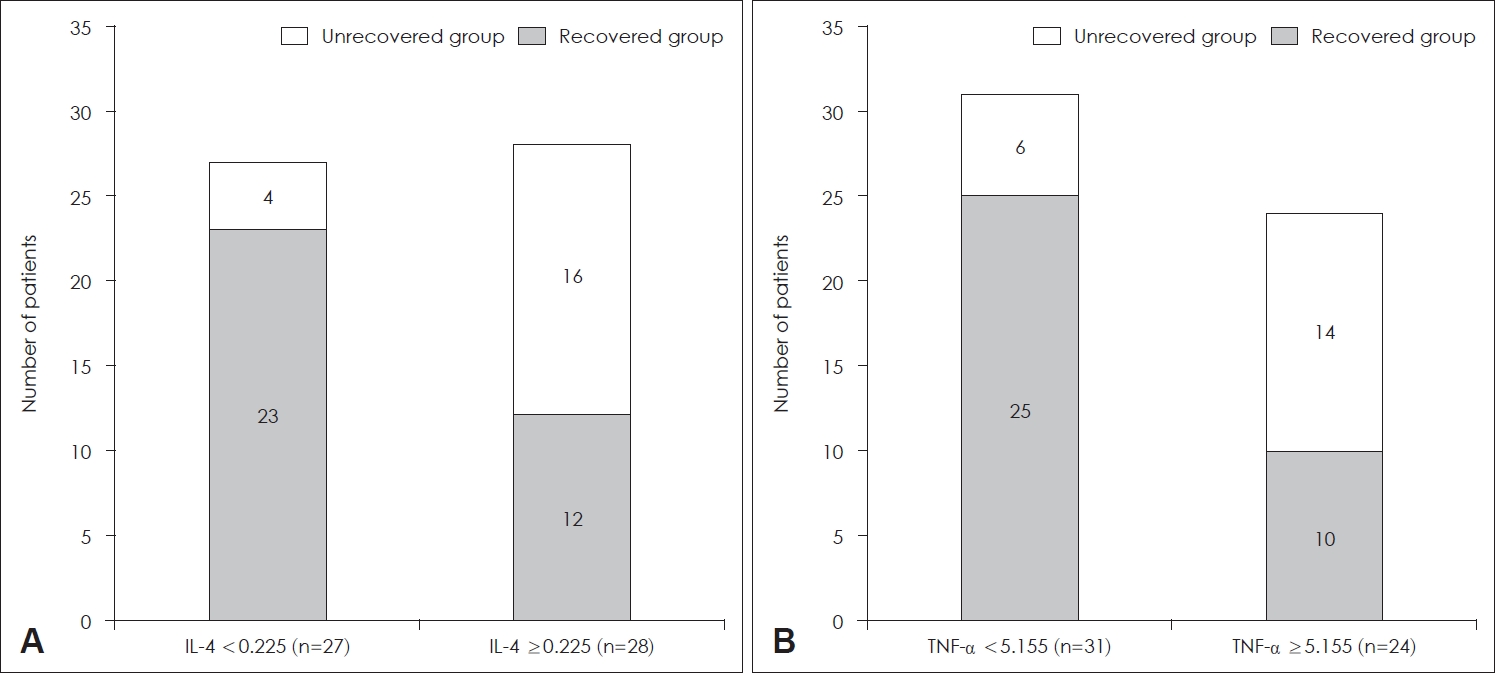
Table┬Ā1.Baseline characteristics of the study population (n=55) Table┬Ā2.The characteristics of the recovered group and unrecovered group (n=55)
Table┬Ā3.Statistical comparisons of cytokine levels in recovered group and unrecovered group (n=55)
Table┬Ā4.Univariate and multivariate logistic regression analysis of clinical variables and cytokine levels for unrecovered group in patients with idiopathic sudden sensorineural hearing loss
REFERENCES1. Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: I. A systematic review. Arch Otolaryngol Head Neck Surg 2007;133(6):573-81.
2. Merchant SN, Adams JC, NadolJ B Jr. Pat hology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otol Neurotol 2005;26(2):151-60.
3. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The proand anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011;1813(5):878-88.
4. Kassner SS, Sch├Čttler S, Bonaterra GA, Stern-Str├żter J, Sommer U, Hormann K, et al. Proinflammatory and proadhesive activation of lymphocytes and macrophages in sudden sensorineural hearing loss. Audiol Neurootol 2011;16(4):254-62.
5. Merchant SN, Durand ML, Adams JC. Sudden deafness: Is it viral? ORL J Otorhinolaryngol Relat Spec 2008;70(1):52-60.
6. Klemm E, Deutscher A, M├Čsges R. A present investigation of the epidemiology in idiopathic sudden sensorineural hearing loss. Laryngorhinootologie 2009;88(8):524-7.
7. Teranishi M, Katayama N, Uchida Y, Tominaga M, Nakashima T. Thirty-year trends in sudden deafness from four nationwide epidemiological surveys in Japan. Acta Otolaryngol 2007;127(12):1259-65.
8. TerBylanishi FM. Seventy-six cases of presumed sudden hearing loss occurring in 1973: Prognosis and incidence. Laryngoscope 1977;87(5 Pt 1):817-25.
9. Seggas I, Koltsidopoulos P, Bibas A, Tzonou A, Sismanis A. Intratympanic steroid therapy for sudden hearing loss: A review of the literature. Otol Neurotol 2011;32(1):29-35.
10. Scherer EQ, Yang J, Canis M, Reimann K, Ivanov K, Diehl CD, et al. Tumor necrosis factor-╬▒ enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke 2010;41(11):2618-24.
11. Tsinaslanidou Z, Tsaligopoulos M, Angouridakis N, Vital V, Kekes G, Constantinidis J. The expression of TNF╬▒, IL-6, IL-2 and IL-8 in the serum of patients with idiopathic sudden sensorineural hearing loss: Possible prognostic factors of response to corticosteroid treatment. Audiol Neurotol Extra 2016;6(1):9-19.
12. Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res 2006;83(4):575-83.
13. Iguchi H, Anniko M. Interleukin 8 can affect inner ear function. ORL J Otorhinolaryngol Relat Spec 1998;60(4):181-9.
14. Ren J, Li H, Lu Y. The determinations of tumor necrosis factor and interleukin 6 in serum of patients with sudden sensorineural hearing loss. Lin Chuang Er Bi Yan Hou Ke Za Zhi 1998;12(7):311-3.
15. Demirhan E, Eskut NP, Zorlu Y, Cukurova I, Tuna G, Kirkali FG. Blood levels of TNF-╬▒, IL-10, and IL-12 in idiopathic sudden sensorineural hearing loss. Laryngoscope 2013;123(7):1778-81.
16. Siegel LG. The treatment of idiopathic sudden sensorineural hearing loss. Otolaryngol Clin North Am 1975;8(2):467-73.
17. Hermann N, Dre├¤en K, Schildberg FA, Jakobs C, Holdenrieder S. Methodical and pre-analytical characteristics of a multiplex cancer biomarker immunoassay. World J Methodol 2014;4(4):219-31.
18. Keithley EM, Wang X, Barkdull GC. Tumor necrosis factor alpha can induce recruitment of inflammatory cells to the cochlea. Otol Neurotol 2008;29(6):854-9.
19. Adams JC. Clinical implications of inflammatory cytokines in the cochlea: A technical note. Otol Neurotol 2002;23(3):316-22.
20. Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inf lammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000;148(2):209-14.
21. Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost 2009;102(2):215-22.
22. Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, et al. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012;379(9822):1205-13.
23. Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 2012;379(9822):1214-24.
24. Nam SI, Ha E, Jung KH, Baik HH, Yoon SH, Park HJ, et al. IL4 receptor polymorphism is associated with increased risk of sudden deafness in Korean population. Life Sci 2006;78(6):664-7.
25. Kelly-Welch AE, Melo ME, Smith E, Ford AQ, Haudenschild C, Noben-Trauth N, et al. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: Expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol 2004;172(7):4545-55.
26. Yamamoto S, Kobayashi I, Tsuji K, Nishi N, Muro E, Miyazaki M, et al. Upregulation of interleukin-4 receptor by interferon-gamma: Enhanced interleukin-4-induced eotaxin-3 production in airway epithelium. Am J Respir Cell Mol Biol 2004;31(4):456-62.
28. Lee YW, Eum SY, Chen KC, Hennig B, Toborek M. Gene expression profile in interleukin-4-stimulated human vascular endothelial cells. Mol Med 2006;10(1-6):19-27.
29. Chen CC, Manning AM. TGF-beta 1, IL-10 and IL-4 differentially modulate the cytokine-induced expression of IL-6 and IL-8 in human endothelial cells. Cytokine 1996;8(1):58-65.
30. Lee YW, Kim PH, Lee WH, Hirani AA. Interleukin-4, oxidative stress, vascular inflammation and atherosclerosis. Biomol Ther (Seoul) 2010;18(2):135-44.
31. Suzuki H, Tabata T, Koizumi H, Hohchi N, Takeuchi S, Kitamura T, et al. Prediction of hearing outcomes by multiple regression analysis in patients with idiopathic sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol 2014;123(12):821-5.
32. Suzuki H, Mori T, Hashida K, Shibata M, Nguyen KH, Wakasugi T, et al. Prediction model for hearing outcome in patients with idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol 2011;268(4):497-500.
33. Cvorovi─ć L, Deric D, Probst R, Hegemann S. Prognostic model for predicting hearing recovery in idiopathic sudden sensorineural hearing loss. Otol Neurotol 2008;29(4):464-9.
34. Moskowitz D, Lee KJ, Smith HW. Steroid use in idiopathic sudden sensorineural hearing loss. Laryngoscope 1984;94(5 Pt 1):664-6.
35. Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol 1977;86(4 Pt 1):463-80.
36. Gianoli GJ, Li JC. Transtympanic steroids for treatment of sudden hearing loss. Otolaryngol Head Neck Surg 2001;125(3):142-6.
37. Zadeh MH, Storper IS, Spitzer JB. Diagnosis and treatment of sudden-onset sensorineural hearing loss: A study of 51 patients. Otolaryngol Head Neck Surg 2003;128(1):92-8.
38. Liu SC, Kang BH, Lee JC, Lin YS, Huang KL, Liu DW, et al. Comparison of therapeutic results in sudden sensorineural hearing loss with/without additional hyperbaric oxygen therapy: A retrospective review of 465 audiologically controlled cases. Clin Otolaryngol 2011;36(2):121-8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |