 |
 |
AbstractBackground and ObjectivesFewer studies are available on geriatric patients’ gustatory dysfunction than on their olfactory dysfunction. Here we aimed to evaluate the relationship between subjective gustatory dysfunction and subjective or objective olfactory dysfunction according to cognitive function in geriatric patients.
Subjects and MethodWe prospectively enrolled patients who underwent both cognitive function test and olfactory function test between August 2018 and May 2019. The correlation between subjective gustatory dysfunction and subjective olfactory dysfunction or conventional olfactory function scores was evaluated for geriatric patients with or withhout cognitive dysfunction. Participants with a threshold-discrimination-identification (TDI) score (<21) on the YSK olfactory function test were diagnosed with olfactory dysfunction. Subjective gustatory function and olfactory function were evaluated using the visual analog scale. The Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet and Mini-Mental State Examination were administered to all participants. Overall, 120 patients (27 male, 93 female; mean age, 73.00±7.50 years) were enrolled.
ResultsWe found that the subjective gustatory function score did not correlate with the threshold, discrimination, identification, or the summation of TDI scores of the olfactory function test but was significantly associated with the subjective olfactory function score (p<0.001). Further, there was no significant correlation between the subjective gustatory function score and cognitive function.
IntroductionOlfactory dysfunction is known to be an early symptom of Alzheimer’s disease (AD) and Parkinson’s disease; however, gustatory dysfunction is not a commonly raised issue in the geriatric field. Previous studies suggested that head injury, sinusitis/upper respiratory infection, increase in medication use among geriatrics are common etiologies in taste dysfunction [1]. Similar to olfactory dysfunction, gustatory dysfunction has been suggested to be associated with neurogenerative disease such as AD [2]. The self-reported olfactory dysfunction rate was reported to be 10.6%, and the gustatory dysfunction rate was 5.3% [3]. As self-reports underestimate the actual prevalence, the exact prevalence of gustatory dysfunction in the geriatric population may be much higher [4].
The relationship between gustatory and olfactory dysfunction is of immense interest. Based on interaction between the sense of taste and smell in production of flavor perception, it is suggested that olfactory dysfunction is closely correlated with gustatory dysfunction. Some studies have reported that patients with gustatory dysfunction are more likely to have underlying olfactory dysfunction [1]. However, other study has reported that olfactory dysfunction has no influence on gustatory dysfunction when the effects of sex, age, and etiology are adjusted for [5].
Cognitive effect also should be regarded in inter-relationship between gustatory and olfactory function because conditioning is important in perception of olfaction and taste. For example, odors such as caramel come to be described as having a “sweet” quality as a result of associations with sugar present in the media in which they are commonly encountered [5].
Therefore, the relationship between gustatory and olfactory dysfunction, and gustatory and cognitive function need to be evaluated. In the current study, we sought to evaluate the relationship between subjective gustatory dysfunction and olfactory dysfunction and also tried to evaluate the impact of cognitive function on subjective gustatory dysfunction in geriatrics.
Subjects and MethodsParticipantsAll experimental protocols were approved by the Institutional Review Board of the Chung-Ang University Hospital (1811-001-309), and written informed consent was obtained from each participant and their caregiver (e.g., spouse or adult child). This prospective study enrolled individuals who agreed to participate in this study between August 2018 and May 2019. All enrolled individuals were aged ≥65 years and underwent the cognitive and olfactory function tests described below. The exclusion criteria were as follows [6]: 1) past or current diagnosis of brain tumor, epilepsy, Parkinson’s disease, major depressive disorder, bipolar disorder, and schizophrenia; 2) past or current diagnosis of neurocognitive disorder due to other conditions except Alzheimer’s and vascular diseases; 3) presence of head trauma or stroke history; and 4) presence of acute rhinitis or sinusitis, active asthma, or a history of obstructive nasal disease or nasal sinus surgery.
Olfactory function testingOlfactory function was tested using the YSK olfactory function test (RHICO Medical Co., Seoul, Korea), which contained three subsets: threshold, discrimination, and identification. The tests were performed according to the manufacturer’s protocol [7]. The sum of the three test scores was calculated as the threshold-discrimination-identification (TDI) score. The score of the threshold test ranged from 1 to 12, while those of the discrimination and identification tests ranged from 0 to 12; the combined TDI score ranged from 1 to 36. Participants with a TDI score lower than 21 were diagnosed with olfactory dysfunction. Subjective gustatory and olfactory dysfunction were evaluated using the visual analog scale.
Neurocognitive testingThe Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K) [8] and the Korean version of the Mini-Mental State Examination (MMSE-K) [9] were administered to all participants by two trained psychiatrists.
Based on the Structured Clinical Interview for DSM-5 Disorders, patients were divided into three groups based on cognitive function: normal, borderline, and impaired.
Statistical analysisAll statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Descriptive data were presented as mean±standard deviation. Pearson correlation was used to evaluate the association between two variables of interest. Inter-group differences were evaluated using one-way analysis of variance. A p-value<0.05 was considered to be statistically significant.
ResultsCharacteristics of enrolled subjectsWe enrolled 120 geriatric patients: 22.50% were male (27/120) and 77.50% (93/120) were female; the mean age was 73.00±7.50 years, and most (98.30%) patients were non-smokers. The mean MMSE-K and CERAD-K scores were 21.56±6.70 and 57.63±22.74, respectively. Of the 120 participants, 31.67% (n=38) and 5.00% (n=6) were diagnosed with neurocognitive disorder due to AD and vascular disease. Based on the results of the olfactory function test (YSK_TDI), 27.50% (n=33) had normosmia, while 72.50% (n=87) had olfactory dysfunction. The subjective gustatory and olfactory function scores were 7.70±1.90 and 6.70±2.30, respectively (Table 1).
Relationship between subjective gustatory function and olfactory function according to cognitive functionWe evaluated the correlation between the subjective gustatory function score and both objective and subjective olfactory function. The subjective gustatory function score did not correlate with the threshold, discrimination, identification, and summation of TDI scores of the olfactory function test (Fig. 1A-D). Instead, the subjective gustatory function score was significantly associated with the subjective olfactory function score (r=0.584, p<0.001) (Fig. 1E).
We then divided the participants into three groups according to their CERAD-K results and compared the corresponding subjective gustatory function scores. There was no significant correlation between the subjective gustatory function score and cognitive function (Fig. 1F).
When we performed univariate and multivariate linear regression analyses, the subjective olfactory function score was the only factor significantly correlated with the subjective gustatory function score (Table 2).
DiscussionDespite the importance of gustatory function in the quality of life, studies about gustatory dysfunction in geriatrics are limited when compared to olfactory dysfunction and the prevalence of gustatory dysfunction is thought to be underestimated.
This is the first study to evaluate the correlation between olfactory and gustatory dysfunction in geriatric patients using the YSK olfactory function test. We found that subjective gustatory function was correlated with subjective olfactory function in the elderly. Thus, our results add another evidence that geriatrics need to performed olfactory function test with gustatory function test together despite gustatory dysfunction not being the first presenting symptom.
When we evaluated whether subjective gustatory dysfunction was related to cognitive function, we found that the presence of AD or an impairment in cognitive function were not associated with subjective gustatory dysfunction. Because patients with neurodegenerative diseases often exhibit eating abnormalities, olfactory and gustatory dysfunctions have been suggested to be involved in these abnormalities. Gustatory function is reported to be adversely affected in the early stages of neurocognitive disorder due to AD [10], and taste scores are significantly reduced among patients with mild cognitive impairment and neurocognitive disorder due to AD [11]. However, we found that the presence of neurocognitive disorder due to AD or cognitive function impairment was not associated with subjective gustatory function.
Theoretically, gustatory perception signals travel via the cranial nerves into the primary gustatory cortex and the insula/frontal perculum. Pathological changes in these areas, such as those observed in semantic dementia, may induce deficits in gustatory function. However, research by Kouzuki, et al. [12] has shown that there is no significant difference in subjective gustatory function and total gustatory test scores between patients with AD, mild cognitive impairment, and healthy controls. Therefore, the relationship between gustatory dysfunction and cognitive function is not conclusive and should be further evaluated.
Interestingly, although subjective olfactory dysfunction was significantly correlated with subjective gustatory dysfunction, there was no correlation found for the TDI score of the YSK olfactory function test. Subjective perception of dysfunction is not always associated with objective test results. Furthermore, olfaction and gustation are closely connected because they share central processing areas, such as the insula and orbitofrontal cortex. Similar to our findings, research has shown that in patients with olfactory dysfunction but not gustatory dysfunction, electric threshold for taste detection, which represents the objective gustatory function, was increased [13]. As it is not easy for patients to clearly distinguish between olfactory and gustatory dysfunction based on subjective symptoms, we suggest the evaluation of both olfactory and gustatory functions in patients presenting with either olfactory or gustatory dysfunction, but not both.
Our study has some limitations. First, we did not perform an objective gustatory function test. In contrast to olfactory function tests, tests that evaluate objective gustatory dysfunction have not been actively developed yet. However, threshold detection regarding sweet, sour, salty, bitter, and umami or electrogustometric taste sensitivity are widely used methods. Further studies with an objective gustatory function test are needed to overcome this limitation. Second, we did not consider parageusia or phantogeusia. It has been suggested that neurological disorders such as Alzheimer’s and Parkinson’s syndrome, which usually present in the elderly, are correlated with olfactory and gustatory hallucinations [14]. We evaluated the subjective recognition of the patient’s taste and olfactory sensitivities. Therefore, considering parageusia or phantogeusia could induce other findings. Finally, we did not consider other factors that can affect the gustatory and olfactory function such as digestive function, gastric emptying and endocrine disorders [14]. We cannot rule out the possibility that these factors have influenced on the relationship between gustatory and olfactory function.
In conclusion, there is a significant relationship between subjective gustatory dysfunction and subjective olfactory dysfunction. As taste perception is important in oral food intake, it is crucial to detect gustatory dysfunction at an early stage and provide an intervention, especially in geriatrics [12]. We suggest that in geriatric patients who present with subjective olfactory dysfunction, gustatory function should be carefully assessed, and an evaluation for the underlying gustatory dysfunction should be actively considered.
NotesAuthor Contribution Conceptualization: Sun Mi Kim, Hyun Jin Min, Kyung Soo Kim, Doug Hyun Han. Data curation Sun Mi Kim, Hyun Jin Min, Seong Hee Kim, Seung Yong Park, Jae Hyoung Choi. Formal analysis Sun Mi Kim, Hyun Jin Min. Investigation Sun Mi Kim, Hyun Jin Min, Kyung Soo Kim, Doug Hyun Han. Methodology Sun Mi Kim, Hyun Jin Min, Kyung Soo Kim, Doug Hyun Han. Project administration Kyung Soo Kim, Doug Hyun Han. Resources Sun Mi Kim, Hyun Jin Min. Supervision Kyung Soo Kim, Doug Hyun Han. Validation Kyung Soo Kim, Doug Hyun Han. Visualization: Sun Mi Kim, Hyun Jin Min, Seong Hee Kim, Seung Yong Park, Jae Hyoung Choi. Writing—original draft: Hyun Jin Min. Writing—review & editing: Hyun Jin Min, Kyung Soo Kim. Fig. 1.Relationship between subjective gustatory dysfunction and olfactory dysfunction. Correlation between the subjective gustatory dysfunction score and YSK olfactory function scores for threshold (A), discrimination (B), and identification (C), as well as with the summation of TDI score (D), and subjective olfactory function score (E). The subjective gustatory dysfunction score was compared with the results of CERAD-K (F). CERAD-K1: normal range; CERAD-K2: significant decline but not dementia level; and CERAD-K3: impaired performance comparable to dementia. TDI, threshold-discrimination-identification; VAS, visual analog scale; CERAD-K, Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet. 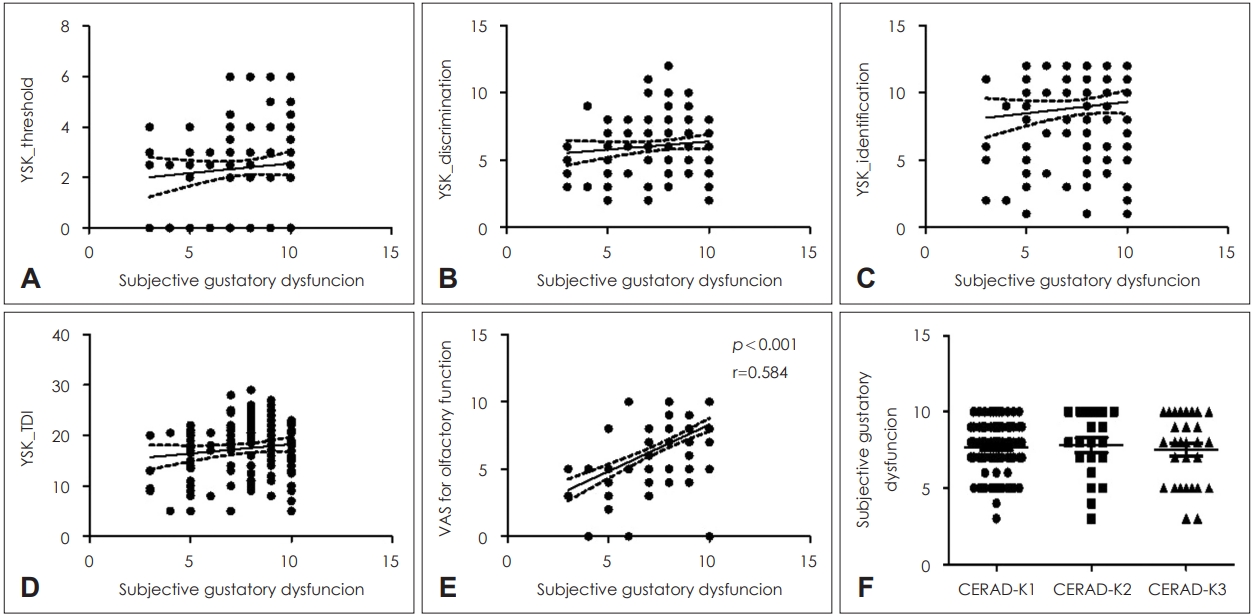
Table 1.Characteristics of the enrolled patients (n=120) Data are presented as mean± standard deviation or n (%). NCD, neurocognitive disorder; AD, Alzheimer’s disease; MMSEK, Korean version of the Mini-Mental State Examination; CERADK, Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet; TDI, threshold-discrimination-identification; VAS, visual analog scale Table 2.Univariate and multivariate logistic regression analyses of clinical characteristics associated with subjective gustatory dysfunction REFERENCES1. Hunt JD, Reiter ER, Costanzo RM. Etiology of subjective taste loss. Int Forum Allergy Rhinol 2019;9(4):409-12.
2. Kai K, Hashimoto M, Amano K, Tanaka H, Fukuhara R, Ikeda M. Relationship between eating disturbance and dementia severity in patients with Alzheimer’s disease. PLoS One 2015;10(8):e0133666.
3. Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. Prevalence and risk factors of self-reported smell and taste alterations: Results from the 2011-2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses 2016;41(1):69-76.
4. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life--an updated review. Chem Senses 2014;39(3):185-94.
5. Stinton N, Atif MA, Barkat N, Doty RL. Influence of smell loss on taste function. Behav Neurosci 2010;124(2):256-64.
6. Kim SM, Kim HR, Min HJ, Kim KS, Jin JC, Han DH. A novel olfactory threshold test for screening cognitive decline among elderly people. PLoS One 2021;16(7):e0254357.
7. Ha JG, Kim J, Nam JS, Park JJ, Cho HJ, Yoon JH, et al. Development of a Korean culture-friendly olfactory function test and optimization of a diagnostic cutoff value. Clin Exp Otorhinolaryngol 2020;13(3):274-84.
8. Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, et al. Development of the Korean version of the consortium to establish a registry for Alzheimer’s disease assessment packet (CERAD-K): Clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci 2002;57(1):P47-53.
9. Park JH, Kwon YC. Standardization of Korean version of the minimental state examination (MMSE-K) for use in the elderly. Part II: Diagnostic validity. J Korean Neuropsychiatr Assoc 1989;28(3):508-13.
10. Sakai M, Kazui H, Shigenobu K, Komori K, Ikeda M, Nishikawa T. Gustatory dysfunction as an early symptom of semantic dementia. Dement Geriatr Cogn Dis Extra 2017;7(3):395-405.
11. Steinbach S, Hundt W, Vaitl A, Heinrich P, Förster S, Bürger K, et al. Taste in mild cognitive impairment and Alzheimer’s disease. J Neurol 2010;257(2):238-46.
12. Kouzuki M, Suzuki T, Nagano M, Nakamura S, Katsumata Y, Takamura A, et al. Comparison of olfactory and gustatory disorders in Alzheimer’s disease. Neurol Sci 2018;39(2):321-28.
|
|
|||||||||||||||||||||||||||||||||||||||

 |
 |