 |
 |
AbstractBackground and Objectives This study analyzes the relationship between endotype biomarkers and the type of tissue inflammation in chronic rhinosinusitis (CRS) in order to obtain a more accurate division of disease groups and appropriate therapy.
Subjects and Method We carried out a cross-sectional study involving 33 samples of CRS patients. We conducted enzyme-linked immunosorbent assay to examine endotype biomarkers and histopathology methods to examine tissue inflammation.
Results Statistical analysis with an independent t-test showed significant differences with respect to eosinophil cationic protein (ECP) between the eosinophilic and neutrophilic groups (p<0.05), whereas there were no significant differences between the two groups (p>0.05) with respect to Charcot-Leyden crystal (CLC), interleukin-5 (IL-5), interferon gamma (IFN-╬│), interleukin- 17A (IL-17A), and oncostatin M (OSM). The Spearman test showed also showed that ECP, CLC, IL-5, IL-17A, IFN-╬│ and OSM had no significant correlation with the two groups (p>0.05). The receiver operating characteristics analysis showed the optimal cut-off value of ECP of 342.22 pg/mL with sensitivity and specificity of 72.7% for determining the inflammation type (area under cover=0.78, p=0.009).
Conclusion The higher level of ECP was more likely to be found in the eosinophilic tissue inflammation. Consequently, we propose using ECP as the main biomarker to identify the CRS type 2. Further research with a larger sample size is required for cut-off points of ECP in order to determine the endotype of CRS.
IntroductionChronic rhinosinusitis (CRS) is a complex disease associated with morbidity and reduced quality of life caused by clinical symptoms of CRS that interfere with individual productivity and rest time. Moreover, CRS is also associated with a high economic burden because it requires long-term medical costs as well as indirect costs caused by the loss of productivity [1]. CRS is a health problem that affects up to 15% of the population in western countries [2]. The CRS epidemiology in various regions also varies widely which might be related to geographic and environmental variations [3]. The understanding of the endotype in CRS has changed the strategy for CRS therapy. However, finding suitable biomarkers which can reliably define type 2 and non-type 2 inflammation to predict response to treatment remains to be a challenge. The CRS endotype depends on the pathophysiological mechanism of the type of tissue inflammation [4]. Studies focusing on endotype biomarkers have recently captured more various inflammation response of CRS based on the cytokine profiles [2]. The number of eosinophils and neutrophils is used as a marker of the type of inflammation. In order to understand the mechanisms underlying CRS, it is important to find out which endotype biomarkers matched the inflammatory type underlying the disease phenotype. Thus, this study was developed to explore biomarkers associated with CRS.
Biomarkers that play an important role in type 2 endotype include interleukin-5 (IL-5), which is the main cytokine in eosinophil differentiation, maturation, toxicity, activation and survival. Eosinophil cationic protein (ECP) is a marker of eosinophil activation and contributes to airway epithelial damage, hypersecretion of goblet cell mucus, and damage to ciliary movement. Charcot-Leyden crystal (CLC) is the main biomarker of eosinophilic inflammation in the airways. CLC can suppress and inhibit T-regulation cell activity thereby affecting immune tolerance during inflammation [5]. Non-type 2 endotypes include type 1 and type 3 inflammation. Type 1 endotype is characterized by the cytokine interferon gamma (IFN-╬│) which induces cell apoptosis. sinonasal epithelium and stimulate neutrophil activity [6]. Type 3 endotype is characterized by the cytokine interleukin-17A (IL-17A) which can modulate neutrophil survival [7]. Oncostatin M (OSM) is a neutrophil product that can interfere with epithelial barrier function by reducing the expression of tight junction protein cells epithelium [8]. There has been no standardized guideline for determining normal values for CRS endotype parameters. Therefore, this research was designed to explore the relationship between endotype biomarkers and the type of tissue inflammation in CRS.
Subjects and MethodsThis was a cross-sectional study between April 2022 and February 2023 in Otorhinolaryngology Head and Neck Surgery Department. This study was approved by the Saiful Anwar Public Hospital Ethical Committee (No. 400/162/K.3/102.7/2022). A written informed consent was obtained from the patients. Inclusion criteria were adult patients over 18 years who met the criteria for the diagnosis of primary CRS based on symptoms and clinical features according to 2020 European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS), characterized by two or more symptoms, one of which should be either nasal blockage or nasal discharge, ┬▒facial pain/pressure, ┬▒reduction or loss of smell, endoscopic findings such as nasal polyps, and/or mucopurulent discharge primarily from middle meatus and/or mucosal obstruction primarily in middle meatus and/or CT scan changes such as mucosal changes within the ostiomeatal complex and/or sinuses. Exclusion criteria were CRS patients that only involved the posterior paranasal sinuses (posterior ethmoid sinus and sphenoid sinus), had previously undergone functional endoscopic sinus surgery (FESS) procedures and had used corticosteroids or antibiotics within four weeks to sample collection. Samples of nasal mucosa were taken with uncinectomy as part of FESS procedure indicated in these patients.
Paraffin block was made using paraffin cutter (Leica Biosystems, Deer Park, TX, USA) Tissue inflammation type of eosinophilic or neutrophilic was determined based on the average number of eosinophils and neutrophils in the lamina propria layer in ten fields of view that have the most infiltration of cells seen under a binocular light microscope CX21 (Olympus, Tokyo, Japan) with 400 times magnification. According to Wicaksana, et al. [9]; the type of tissue inflammation can be assessed based on the ratio neutrophil eosinophil (RNE) which is defined as the ratio between the average number of neutrophils and the average number of eosinophils. If the RNE is more than 2.8 it indicates neutrophilic tissue inflammation and the RNE is less than equal to 2.8 it indicates eosinophilic inflammation.
The endotype of the tissue was determined using several biomarkers. The type 2 endotype was marked with CLC and/or ECP and/or IL-5 biomarkers. While the non-type 2 endotypes characterized by the IFN-╬│, IL-17A and OSM biomarkers. The CLC, ECP, IL-5, IFN-╬│, OSM and IL-17A mucosal tissues of the uncinate process of CRS patients were examined by enzyme-linked immunosorbent assay (ELISA) method. The ELISA Kit 96T CLC, ECP, IL-5, IFN-╬│, OSM dan IL-17A from Finetest and Microplate reader (ZENIX-320; Zenix, Taoyuan, Taiwan) were used in this study. The data obtained from the ELISA examination is in the form of numbers, to determine the normal value limit it will be analyzed statistically using the receiver operating characteristics (ROC) curve. All of the sample was processed and analyzed in the anatomical pathology laboratory by an anatomical pathologist.
Statistical analysis for comparison between endotype biomarkers in each group of tissue inflammation types will be analyzed using an independent t-test. The correlation between endotype biomarkers and the type of tissue inflammation was analyzed using Spearman, both for type 2 and non-type 2 endotypes. The results of the data analysis will be made in categorical form by constructing a ROC curve to determine the cut off of endotype biomarkers. The relationship between endotype biomarker data and the type of tissue inflammation in chronic rhinosinusitis will be analyzed by chi-square. Risk factor is calculated with a limit if >1 is considered a risk factor. A 95% confidence interval with p<0.05 was considered to be statistically significant. All analysis was performed with SPSS version 25 for Mac (IBM Corp., Armonk, NY, USA).
ResultsA total of 33 patients with primary chronic rhinosinusitis was included in this study the mean age of 38.4┬▒15.3 years. There were 12 male and 21 female included in this study. The main symptoms observed among the patients were nasal obstruction, rhinorrhea, and facial pain. The mean symptoms duration was 91.2┬▒110 weeks. The demographic and clinical characteristics details can be seen in Table 1.
There was a higher number of females in the eosinophilic type (72.7%) compared to the neutrophilic type (45.5%), however, there was no statistically significant difference between the groups. The age range for the inflammatory type of eosinophilic tissue was 51 (18-68) years while for the inflammatory type of neutrophilic tissue was 28 (20-56) years and there was no significant difference in age criteria between the two types of inflammation. The complaints experienced by the eosinophilic inflammatory type group were more varied. While, the neutrophilic type showed nasal blockage and rhinorrhea However, there was no significant difference in main symptoms between the two groups The symptom duration range in the eosinophilic and neutrophilic inflammatory type group ranged from 36 (12-416) and 52 (12-416), respectively. There was no significant difference based on the duration of symptoms. There was no difference in the biomarker expression of CLC, IL-5, IFN-╬│, IL-17A, and OSM between the eosinophilic and neutrophilic groups. The significant difference in the type 2 endotype biomarker of ECP was observed between the eosinophilic type (392.68┬▒130.21 pg/mL) and neutrophilic type (274.59┬▒103.22 pg/mL). Further details can be seen in Table 1. The comparison of the biomarkersÔÇÖ expression based on the inflammation types can be seen in Fig. 1.
There was no correlation between ECP, CLC, IL-5, IFN-╬│, IL-17A, and OSM with eosinophilic type inflammation. Similar result also found for the correlation of ECP, CLC, IL-5, IFN-╬│, IL-17A, and OSM with neutrophilic type inflammation. The result of correlation analysis can be seen in Table 2.
The ROC analysis showed the optimal cut-off value of ECP of 342.22 pg/mL with a sensitivity and specificity of 72.7% for determining the inflammation type (area under cover=0.78, p=0.009) (Fig. 2). Based on the cut-off value, the analysis of the association between the ECP and inflammation type showed a higher proportion of ECP biomarkers more than 342.22 pg/mL in the eosinophilic group compared to neutrophilic group. However, it was not statistically significant (p=0.46). The crosstabulation between the inflammation type and ECP with determined cut-off can be seen in Table 3.
DiscussionThe eosinophilic inflammation was predominantly found in females, while neutrophilic inflammation have a relatively similar proportion of male and females subjects. From the available data, heterogeneity of sex prevalence in CRS cases was obtained which was influenced by several factors such as environmental, hormonal and geographical. However, further studies are needed to determine the possible role of psychosocial, cultural and economic factors responsible for gender differences [10]. The duration was related to the severity and the patients behavior to seek medical treatment. Based on a study conducted by Kato, et al. [11]; where eosinophilic inflammation was associated with high recurrence and more severe disease severity in both CRS with and without nasal polyps. In this study, the main complaints were dominated by nasal congestion or blockage in both the neutrophilic and eosinophilic groups. According to the 2020 EPOS, the diagnostic criteria based on the main symptoms of CRS are nasal congestion and rhinorrhea [12], where these two complaints are also complaints that are often experienced in the subjects of this study.
This study found that ECP in the eosinophilic group was significantly higher than the neutrophilic group. The ECP alone is considered an accurate marker of eosinophil inflammation. This is supported by several studies such as research conducted by Yang, et al. [13]; who found that eosinophilic inflammation characterized by ECP can cause downregulation of transforming growth factor (TGF) which is one of the biomarkers of neutrophilic inflammation markers and conversely neutrophilic inflammation can release IL-8 which causes an increase in TGF expression. This can also be seen based on the histological picture in CRS patients where there is tissue edema with eosinophil infiltration which is characterized by the increased in the ECP expression. Research conducted by Bachert, et al. [14]; found that there was a strong relationship between IL-5 and ECP which is a marker of eosinophil activation. In this study, it was found that there was a significant difference between ECP levels in the eosinophilic group and the neutrophilic group. However, the increase in ECP biomarker levels was not followed with the increase in the number of eosinophils in the tissue [15]. This occurs because eosinophils undergo degranulation and produce ECP, the morphological characteristics of these eosinophils will be different from those of eosinophils in general, so they have the potential to be undetectable by hematoxylin eosin staining.
Studies regarding the ECP cut-off value associated with eosinophils in sinus tissue are very limited. ECP is more often used as a ratio compared to myeloperoxidase to define the type of inflammation or used to compare two groups of endotypes. However, there is a study conducted by Lou, et al. [16]; stating that ECP is a biomarker that is considered more accurate in assessing eosinophil infiltration, where in this study ECP levels were assessed based on previous research conducted by Tan, et al. [17]; using 95th percentile of preexisting control of ECP. The results found different ECP levels in the two control groups, namely >131.5 ng/mg and >289.75 ng/mg. This was differed with our result for cut-off value of 342.22 pg/mL with 42.4% accuracy. This difference indicates that ECP levels have various values in the control group and the number of samples also affects the cut-off value. Therefore, a multicentre study is needed to determine criteria that can be used in general in determining biomarker cut-off in CRS endotypes.
In this study, there was no significant difference of the CLC levels between the eosinophilic and neutrophilic groups, which might be explained by the ability of CLC to induce neutrophil activation. The study conducted by Delemarre, et al. [18]; found that there was a relationship between CLC and the induction and process of neutrophilic inflammation in CRS with nasal polyps. In addition, this study also found that there was no correlation between IL-17 levels and the neutrophil count of CRS patients with nasal polyps. The neutrophilic protease elastase and cathepsin G were increased in CRS with type 2 endotype. This suggests that neutrophils are not only more frequently found in type 2 endotypes, but also have a higher potential to influence local inflammation through increased proteolytic activity. Elastase and cathepsin G are known to increase the secretion and activation of IL-1 and IL-33 cytokines, these cytokines are the main cytokines in the induction of type 2 responses because they function in activation of T-helper 2 (Th2) cells and stimulate the production of type 2 endotype cytokines in eosinophilic type nasal polyps [18]. Moreover, the increased in neutrophilic inflammation might be related to bacterial colonization and the variation in the composition of the sinus bacterial microbiota which was shown to be associated with CRS heterogeneity. Thus, these factors may contribute to the inflammation type of CRS endotypes.
In this study, we found no significant difference in the IL-5 levels in the eosinophilic and neutrophilic groups. The similar result was also found in a study conducted in New Zealand by Hoggard, et al. [19]; that the IL-5 levels were equally elevated in the eosinophilic and non-eosinophilic groups. This might be caused by the difference in the geographical factors. In addition, Delemarre, et al. [2]; also found that there was IL-5 and IL-9 expression in tissue neutrophils in CRS patients with nasal polyps accompanied by asthma. This shows that neutrophils had the potential to initiate a type 2 response which can be the basis for eosinophil infiltration.
There was no significant differences in IL-17A levels in the eosinophilic and neutrophilic groups in this study. This was also found by several previous studies. In the study conducted by Cho, et al. [20]; found that the inflammatory group of eosinophilic tissues showed unique characteristics compared to other CRS subtypes and control tissues, that is, when adjusted for atopic status, smoking history, and duration of disease, the IL-17A biomarker increased continuously with the increasing age. However, Kim, et al. [21]; found that the neutrophil-associated cytokines such as IL-17A and IL-23 significantly decreased with age. This might be explained by the differences in the characteristics of eosinophilic inflammation between Asian countries and Western countries.
IFN-╬│ is a characteristic marker of type 1 inflammation and is activated by Th1 cells. IFN-╬│ will induce apoptosis of sinonasal epithelial cells and stimulate neutrophil activity [6]. In non-type 2 inflammation, epithelial cells will secrete osteopontin (OPN). The OPN-stimulated dendritic cells will induce Th1 and Th17 which regulate non-eosinophilic inflammation through the production of IFN-╬│, IL-17A. The IFN-╬│ can induce apoptosis of epithelial cells [14]. In this study there were no significant differences in IFN-╬│ levels in the eosinophilic and neutrophilic groups. This was also found in a study in Americans in Chicago which failed to prove the regulation of IFN-╬│ in CRS patients without nasal polyps. The differences may be due to the presence of different endotypes in different geographic areas. A multicenter study involving subjects from different continents found that IFN-╬│ levels were significantly higher in patients with CRS without nasal polyps than in CRS patients with nasal polyps but this was only true in Beijing but not in the other 5 regions [22]. Wang, et al. [23]; confirmed that there was increased expression of IFN-╬│ and IL-17A in CRS patients in both eosinophilic and non-eosinophilic inflammatory groups in Wuhan compared to control subjects, but the non-eosinophilic group had higher IFN-╬│ and IL-17A expression compared to the eosinophilic group.
OSM is part of the IL-6 cytokine produced by neutrophils which can interfere with epithelial barrier function by reducing the expression of tight junction protein epithelial cells. A study conducted by Pothoven, et al. [24]; found that OSM increased in the sinonasal tissue of CRS patients with rice polyps. In addition, they also found that OSM in tissues correlated with the level of ╬▒2-macroglobulin which is a marker of sinonasal mucosal epithelial leakage. This suggests that OSM might mediate epithelial barrier dysfunction in CRS. Further research identified that neutrophils are the main OSM-producing cells in nasal polyps. Similarly, a study conducted by Tian, et al. [25]; also revealed that the expression level of OSM was increased in CRS and could be the main cause of disruption of the integrity of the mucosal barrier in the pathogenesis of CRS. Epithelial cells, such as keratinocytes, are target cells for OSM and work by inhibiting cell differentiation and inducing chemokines and antimicrobial peptides. However, our study found no significant difference in OSM levels in the eosinophilic and neutrophilic groups, but OSM levels tended to be higher in the eosinophilic group. There was neutrophil activation causing high OSM levels in the eosinophilic group because the OSM was produced by neutrophils. This finding was similar to the study by Poposki, et al. [26]; who reported that the cytokine OSM which disrupts the epithelial barrier increases in eosinophilic inflammatory types in CRS with nasal polyps in the United States and states that neutrophils are the main source of OSM in nasal polyps.
In this study, there were limitations such as abnormal data distribution because there were several levels of endotypes that had extreme values. This can be seen from some of the biomarker endotype data which has a standard deviation that is greater than the average. Another limitation in this study is the difficulty of validating the effectiveness of endotype biomarkers because they are highly influenced by individual factors and environmental factors that underlie inflammation of the paranasal sinus mucosa, so caution is required in the interpretation and application of each endotype biomarker research results in different region. Research is needed on the value of endotype biomarkers in each region to find out how far environmental and individual factors affect these biomarker values and it is hoped that in the future a basic biomarker value can be obtained that can be used as a guideline. In this study there was also a limited research time so that the sample was taken by non-random sampling.
Although there was no significant association between ECP endotype biomarkers and the type of tissue inflammation based on predetermined cut-offs, the ECP hold a potential as a biomarker for type 2 endotype for its possible association with eosinophilic type of inflammation. We propose using ECP as the main biomarker to identify CRS type 2. The CRS endotyping based on the tissue inflammation response which may be influenced by various factors should be replaced with more specified biomarkers. Further research with larger sample size is required to have the cut-offs point of ECP in order to determine the endotype of CRS.
NotesAuthor Contribution Conceptualization: Iriana Maharani, Monica Intan. Data curation: all authors. Formal analysis: all authors. Methodology: all authors. Validation: all authors. WritingÔÇöoriginal draft: Iriana Maharani, Monica Intan. WritingÔÇöreview & editing: all authors. Fig.┬á1.The chart diagram showing the comparison of biomarkers expression in eosinophilic and neutrophilic chronic rhinosinusitis. There were significant differences in the ECP type 2 endotype biomarkers, while the other biomarkers did not have significant. OSM, oncostatin M; IL, interleukin; IFN-╬│, interferon gamma; CLC, Charcot-Leyden crystal; ECP, eosinophil cationic protein. 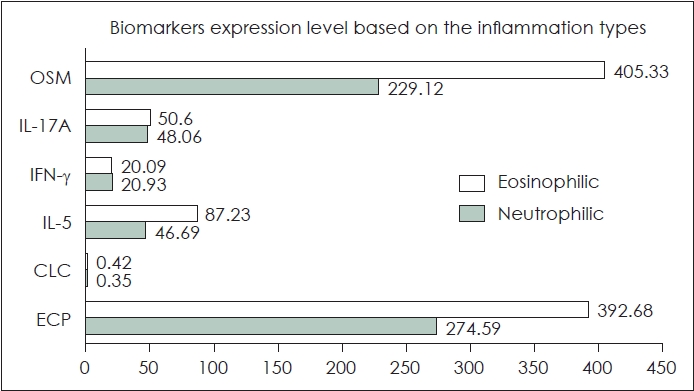
Fig. 2.The receiver operating characteristics curve of eosinophil
cationic protein biomarker. Area under cover=0.78, p=0.009. Sensitivity and specificity of 72.7%. 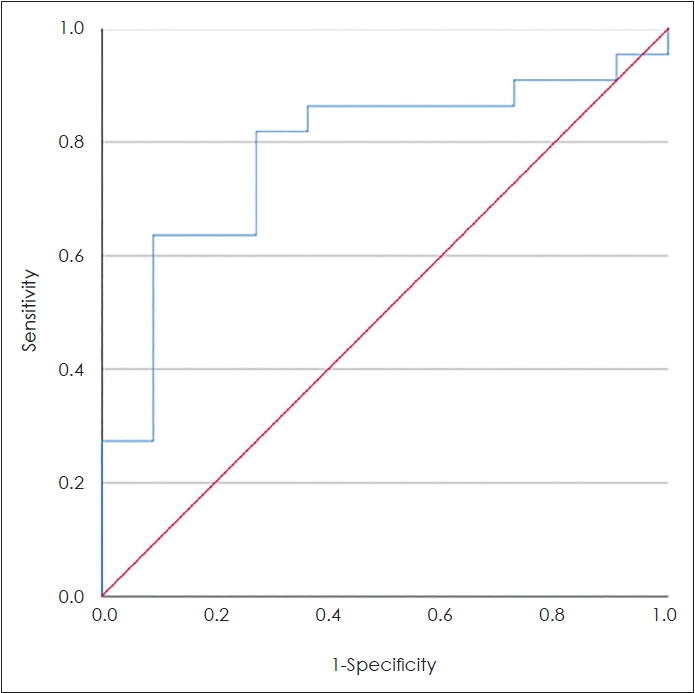
Table 1.Demographics and endotyping biomarkers of patients with primary chronic rhinosinusitis and characteristics based on the inflammation type
Table 2.The correlation analysis of endotype biomarkers with the eosinophilic and neutrophilic inflammation type REFERENCES2. Delemarre T, Bochner BS, Simon HU, Bachert C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J Allergy Clin Immunol 2021;148(2):327-35.
3. Talat R, Speth MM, Gengler I, Phillips KM, Caradonna DS, Gray ST, et al. Chronic rhinosinusitis patients with and without polyps experience different symptom perception and quality of life burdens. Am J Rhinol Allergy 2020;34(6):742-50.
4. Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Chronic rhinosinusitis: Pathogenesis, therapy options, and more. Expert Opin Pharmacother 2018;19(16):1805-15.
5. Liu C, Yan B, Qi S, Zhang Y, Zhang L, Wang C. Predictive significance of CharcotÔÇôLeyden crystals for eosinophilic chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy 2019;33(6):671-80.
6. Xu X, Ong YK, Wang Y. Novel findings in immunopathophysiology of chronic rhinosinusitis and their role in a model of precision medicine. Allergy 2020;75(4):769-80.
7. Huriyati E, Darwin E, Yanwirasti Y, Wahid I. Differences in expression of inflammatory mediator in mucosal and polyp tissue between chronic rhinosinusitis and recurrent chronic rhinosinusitis. Open Access Maced J Med Sci 2019;7(11):1733-8.
8. Ahern S, Cervin A. Inflammation and endotyping in chronic rhinosinusitisÔÇöa paradigm shift. Medicina (Kaunas) 2019;55(4):95.
9. Wicaksana DA, Suheryanto R, Maharani I. Correlation between ╬▓-glucan and neutrophil/eosinophil ratio of paranasal sinus mucosa and blood in the diagnosis of chronic fungal rhinosinusitis. Oman Med J 2018;33(5):393-400.
10. Brescia G, Contro G, Ruaro A, Barion U, Frigo AC, Sfriso P, et al. Sex and age-related differences in chronic rhinosinusitis with nasal polyps electing ESS. Am J Otolaryngol 2022;43(2):103342.
11. Kato A, Peters AT, Stevens WW, Schleimer RP, Tan BK, Kern RC. Endotypes of chronic rhinosinusitis: Relationships to disease phenotypes, pathogenesis, clinical findings, and treatment approaches. Allergy 2022;77(3):812-26.
12. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. Executive summary of EPOS 2020 including integrated care pathways. Rhinology 2020;58(2):82-111.
13. Yang LY, Li X, Li WT, Huang JC, Wang ZY, Huang ZZ, et al. V╬│1+ ╬│╬┤T cells are correlated with increasing expression of eosinophil cationic protein and metalloproteinase-7 in chronic rhinosinusitis with nasal polyps inducing the formation of edema. Allergy Asthma Immunol Res 2017;9(2):142-51.
14. Bachert C, Zhang N, Hellings PW, Bousquet J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol 2018;141(5):1543-51.
15. Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med 2015;192(6):682-94.
16. Lou H, Zhang N, Bachert C, Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol 2018;8(11):1218-25.
17. Tan BK, Klingler AI, Poposki JA, Stevens WW, Peters AT, Suh LA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol 2017;139(2):699-703.e7.
18. Delemarre T, Holtappels G, De Ruyck N, Zhang N, Nauwynck H, Bachert C, et al. A substantial neutrophilic inflammation as regular part of severe type 2 chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2021;147(1):179-88.e2.
19. Hoggard M, Waldvogel-Thurlow S, Zoing M, Chang K, Radcliff FJ, Wagner Mackenzie B, et al. Inflammatory endotypes and microbial associations in chronic rhinosinusitis. Front Immunol 2018;9:2065.
20. Cho SH, Kim DW, Lee SH, Kolliputi N, Hong SJ, Suh L, et al. Age-related increased prevalence of asthma and nasal polyps in chronic rhinosinusitis and its association with altered IL-6 trans-signaling. Am J Respir Cell Mol Biol 2015;53(5):601-6.
21. Kim DK, Kim JY, Han YE, Kim JK, Lim HS, Eun KM, et al. Elastase-positive neutrophils are associated with refractoriness of chronic rhinosinusitis with nasal polyps in an Asian population. Allergy Asthma Immunol Res 2020;12(1):42-55.
22. Cao PP, Wang ZC, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol 2019;122(1):33-40.
23. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol 2016;138(5):1344-53.
24. Pothoven KL, Norton JE, Hulse KE, Suh LA, Carter RG, Rocci E, et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J Allergy Clin Immunol 2015;136(3):737-46.e4.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |