성문하 및 성문후 기도협착 소아 환자에서 개방 기도 재건술의 실패 원인 분석
Analysis of Failure Causes of Open Airway Reconstruction in Children With Combined Subglottic and Posterior Glottic Stenosis
Article information
Trans Abstract
Background and Objectives
Airway reconstruction surgery in children is still challenging, especially in cases of combined subglottic and posterior glottic stenosis (PGS). The aim of this study was to review the underlying reasons of failure in open airway reconstruction surgeries performed for children with combined subglottic and PGS.
Subjects and Method
We reviewed medical records of seven children who received more than two open airway reconstruction surgeries to finally achieve and maintain decannulation status for more than one year. Twenty-two reconstructive surgeries were performed and they consisted of 19 laryngotracheal reconstruction (LTR), 2 cricotracheal resection with end-to-end anastomosis (CTR) and one extended CTR. For each patient, the following potential causes of failure were evaluated; preoperative evaluation (PE), type of reconstruction (TR), single vs. double staging (SDS), type of stent (TS), and perioperative optimization (PO).
Results
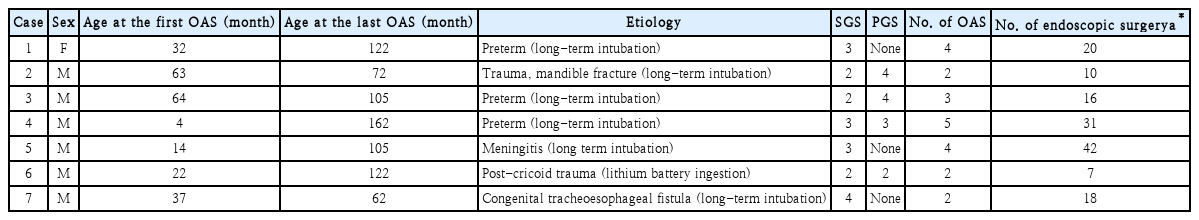
The median age of patients at the time of surgery was 32 months (range, 4-64 months). Successful decannulation was achieved after the median open surgery of three (range, 2-5 times for each patient). Recognized causes of failure were as follows: 8 insufficient PE, 10 inadequate TR, 3 improper SDS, 8 ill-chosen TS, and 2 inappropriate PO.
Conclusion
PE of dynamic airway is important, especially vocal fold mobility and tracheomalacia. Types of reconstruction should be carefully decided after full consideration of potential causes of failure, and adequate laryngotracheal stent is essential.
Introduction
Surgery for pediatric airway stenosis is constantly evolving, and a variety of endoscopic and open surgical approaches have been described. Nevertheless, surgical interventions for laryngotracheal stenosis (LTS) in children remain challenging and stressful for pediatric surgeons and otolaryngologists [1]. The success rate of endoscopic balloon dilation for LTS is reported to be up to 90% [2], and that of open reconstructive surgery is up to 85% [3]. However, most previous studies focused on patients with isolated subglottic stenosis or low-grade stenosis. Although endoscopic surgery is frequently used as primary therapy for grade I or II subglottic stenosis, overenthusiastic use of laser treatment and dilation procedures may worsen the extent of the initial stenosis, and recurrence rates can be 70% over a 5-year follow-up [4]. Open surgery is usually required for patients with advanced stage of subglottic stenosis [2,5-8]. The two most commonly performed open procedures are laryngotracheal reconstruction (LTR) and cricotracheal resection with end-to-end anastomosis (CTR). LTR includes a spectrum of procedures; splitting the anterior cricoid splitting (aCS) and/or posterior cricoid splitting (pCS), with or without interposition of a cartilage graft (CG). Pediatric CTR is one of the major surgical options for the treatment of moderate to severe subglottic stenosis.
The most common cause of synchronous subglottic and posterior glottic stenosis (PGS) is prolonged intubation during mechanical ventilator care [9]. The traditional surgical approach for a combined stenosis have been LTR and placement of anterior and posterior CGs. Vocal cord fixation and interarytenoid stenosis are meaningful predictive factors associated with failure of LTR [10]. In cases of subglottic stenosis with interarytenoid adhesion, CTR and extended CTR (CTR and pCS with CG) provide better results than those of simple LTR with CG [11].
In this study, we reviewed our experience of open airway reconstructions in children with combined subglottic and PGS. We evaluated the causes of failure in those patients who received multiple reconstructive surgeries for combined subglottic and posterior glottis stenosis to achieve successful decannulation.
Subjects and Methods
Subjects
This retrospective study of case series was approved by Institutional Review Board of the Samsung Medical Center (Approval No. 2017-09-009). We investigated the pediatric patients who had multiple airway reconstruction surgery for combined subglottic and posterior glottis stenosis to analyze the causes of failure of open airway reconstruction surgeries. We enrolled 7 children (6 males and 1 female) who received multiple LTR and/or CTR at our institution from 1998 to 2017. Twenty-two open airway reconstruction surgeries were performed for these 7 patients. Patients who fulfilled the following inclusion criteria were included in the study:
1) Combined subglottic and posterior glottis stenosis
2) Two or more open airway reconstruction surgeries until decannulation
3) Age at the time of surgery <18 years old
Demographic data including age, sex, cause of the LTS, height, and body weight were collected. Grade of subglottic stenosis before each airway reconstruction surgery was determined with the Myer-Cotton Airway Grading System; grade I corresponds to ≤50% airway obstruction; grade II to 51%-70%; grade III to 71%-99%; and grade IV to no detectable lumen [12]. PGS were described according to the BogdasarianOlson classification; grade I is interarytenoid synechia; grade II is posterior commissure stenosis; grade III is posterior commissure stenosis with one cricoarytenoid joint ankylosis; and grade IV is posterior commissure stenosis with bilateral cricoarytenoid joint ankyloses [13]. Preoperative evaluation (PE) was mostly performed under general anesthesia. Mobility of the vocal folds were determined by palpation of the arytenoid cartilages. Whenever possible, fiberoptic or rigid endoscopic evaluation was performed at the outpatient clinic in children who could cooperate. All patients had tracheostomy cannula prior to the open airway reconstructive surgery.
Surgical procedures
The types of reconstruction were categorized into LTR, CTR, and extended CTR (eCTR) [14]. The costal cartilage was harvested for the free-graft reconstruction of LTR. The graft was oriented in place with the perichondrium facing the airway lumen. Posterior graft was designed to have a rectangular shape with beveled shelves. Anterior graft was designed to have a fusiform or modified boat-shape with rib edges left intact as flanges to prevent collapse of the graft into the airway. For CTR, stenotic upper trachea and anterior half of the cricoid ring was resected. The remaining posterior cricoid plate was thinned using a drill burr. The posterior mucosa of distal trachea was designed as a tongue-like shape and advanced to cover the thinned cricoid plate. For eCTR, subglottic stenosis lesion was resected and posterior cricoid plate was divided in the midline and posterior laryngotracheal structures are widened using CG. CG was fixed with 4-0 Vicryl, and release of distal trachea was performed. Exposed cartilage and graft were covered with normal mucosa and thyrotracheal anastomosis was completed.
According to the timing of tracheostoma closure, a single-stage operation was defined when orotracheal intubation was maintained with closure of the tracheostoma at the time of reconstruction. Patients with single-stage surgery were kept intubated at the intensive care unit (ICU) for 5-7 days, and extubation was tried at the operation room. A double-stage surgery was defined when the tracheostomy tube was kept in place at the end of the surgery. For the double-stage reconstruction, Montgomery T-tube or Montgomery laryngeal stent was used, or stent was not used in some cases. Most of the patients underwent several ancillary endoscopic procedures including incision of scar tissue, balloon dilation, or removal of granulation tissue after the reconstruction surgery.
Analysis of failure
Successful “decannulation” was defined as the removal of tracheostomy cannula with closure of the tracheostoma and without further open airway surgery for more than 12 months. Potential causes of decannulation failure were listed according to the review of previous literature [15]. Four head and neck surgery specialists individually reviewed the medical records and marked the reasons of failure among the lists; PE, type of reconstruction (TR), single vs. double staging (SDS), type of stent (TS), and perioperative optimization (PO). Afterwards, they discussed together to reach the consensus. PE included endoscopic examination, CT, and X-ray to identify stenotic lesion and vocal fold mobility. TR was about whether an appropriate surgical method was selected according to the status of LTS. SDS was about appropriate selection for the presence or absence of tracheostoma at the time of airway reconstruction surgery. PO included management of underlying disease, wound care, and postoperative general management.
Results
Patient characteristics and cause of failure of each airway surgery
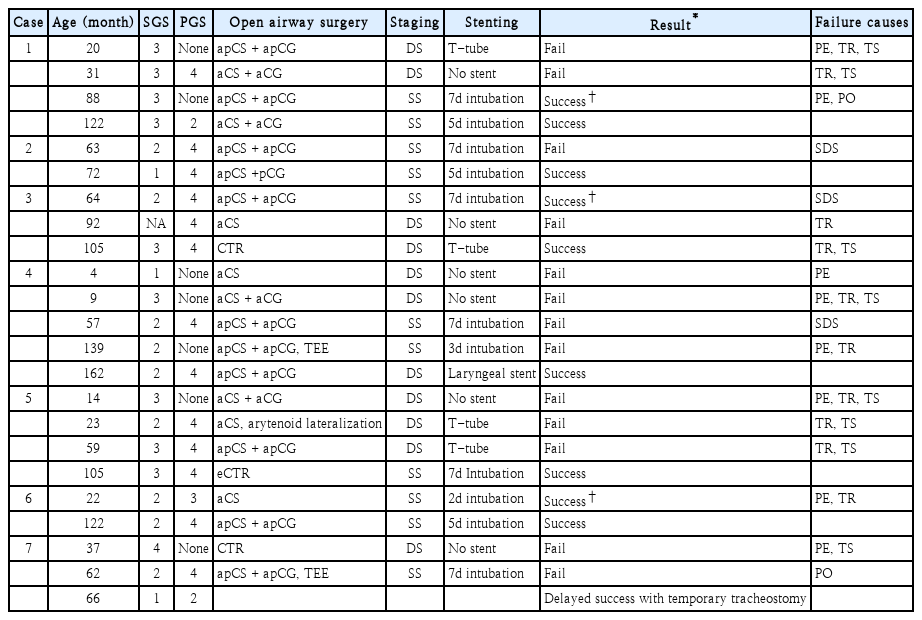
The demographic and clinical characteristics were summarized in Table 1. Case numbers were assigned according to the chronological order of initial reconstructive surgery. Details of surgical interventions for each patient were summarized in Table 2. For example, case #1 was a 20-month-old female at the time of first open airway surgery. Double-stage LTR with anterior and posterior cricoid splitting (apCS) with cartilage graft (apCG) was initially performed. Vocal fold mobilities were not precisely evaluated before the first surgery. Montgomery T-tube was used as a stent. As potential reasons of failure of the first surgery, insufficient PE, inadequate TR, and ill-chosen TS were pointed out. A correct diagnosis would be a combined subglottic grade 3 and PGS grade 4. A recommended surgery would be a double-staged eCTR with the use of Montgomery laryngeal stent. She received total 4 open airway surgeries to get finally decannulated, which took about 117 months (9.75 years) after her tracheostomy. One or several reasons of failure were listed for each surgery. In summary, recognized causes of failure were as follows; 8 insufficient PE, 10 inadequate TR, 3 improper selection of single or double stage surgery, 8 ill-chosen TS, and 2 inappropriate POs.
Surgical outcomes according to the types of surgery
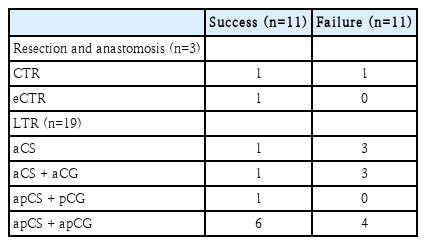
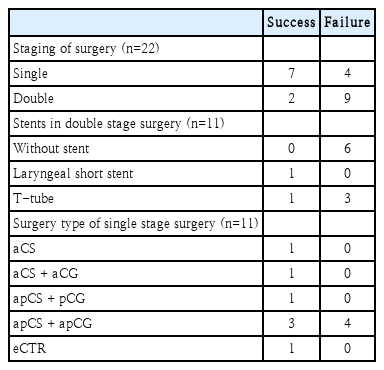
Outcomes according to the type of surgery is summarized at Table 3. There were 1 success (case #3) and 1 failure (case #7) with CTR. A case of eCTR was performed to be successful (case #4). For aCS and aCS with aCG, both types of surgery showed 1 success and 3 failures. In cases of apCS with apCG, there were 6 successes and 4 failures. We also investigated surgical outcomes according to the SDS, the TSs in double stage, and the type of surgery in single stage surgery (Table 4). Single stage was successful in 7 of 11 surgeries. Double stage was successful in 2 of 11 surgeries. Regarding 11 double stage surgeries, success rate was 0% for no stent use (n=0/6) and 25% for Montgomery T-tube stent (n=1/4), and 100% for a laryngeal short stent (n=1/1). Regarding 11 single stage surgeries, success rate was 60% for LTR (n=6/10) and 100% for eCTR (n=1/1).
Discussion
A study of the most experienced centers reported the treatment outcomes of 33 children with combined subglottic and PGS [16]. According to their reports, the overall decannulation rate was 79% and the operation-specific decannulation rate was 61% [16]. In our study, all of the enrolled patients finally achieved successful decannulation. However, the operation-specific decannulation rate was 40.9% (9/22). The discrepancy in operation-specific success rate between the previous reports and our results probably resulted from differences in the well-established clinical pathways as well as the experiences of surgeons. Over the 20 years of study period (1998-2017), multiple airway reconstructions were performed in 7 patients at the authors’ institution. At the earlier period, clinical pathways for those patients were not properly set up and the authors’ experiences were insufficient to precisely manage complex multilevel airway stenosis in children.
Among the recognized causes of failure in our study, inadequate TR (n=10) was most common, which was followed by insufficient PE (n=8), ill-chosen TS (n=8), and improper single or double stage surgery (n=3). In fact, the reasons of failure were closely related with each other. Until recently, we could not evaluate the dynamic airway under general anesthesia while keeping patients’ self-respiration. Therefore, degree of vocal fold mobility and malacia (supraglottic or tracheal) could not be precisely evaluated in younger children who were not able to cooperate laryngoscopic evaluation in outpatient clinics. Recently, many physicians including our clinic have tried to perform dynamic airway evaluation with high flow ventilation without intubation, During high flow ventilation, we could evaluate airway status more accurately with sedative agents.
Insufficient PE unsurprisingly resulted in inadequate choices of TR; aCS±aCG were performed but failed in 6 cases of combined subglottic and grade 3 or 4 PGS. If vocal fold mobilities were properly evaluated in those cases to be severely limited or fixed, apCS with apCG or extended CTR would be an ideal choice of intervention according to the severity of subglottic stenosis.
Unfortunately, availability of laryngo-tracheal stents has been limited in the authors’ area. Montgomery laryngeal stents and T-tubes are no longer officially imported because of the distributor problems. Other commercially available laryngotracheal stents are not available including Monnier laryngotracheal molds. Because of these unavailability, laryngeal stent was not placed even in double stage LTR (n=4). In another 4 cases, Montgomery T-tube should be removed earlier than planned schedule because of intraluminal obstruction with thick secretion, crust formation, or granulation formation. In 3 cases of apCS with apCG, single stage LTR was tried but failed. If appropriate laryngeal stents were available at that time, staged LTR might increase the possibility of success since those children had combined grade 4 posterior glottic and grade 2-3 subglottic stenosis. Based on our experiences and review of literatures, we suggested the clinical pathway for the management of combined subglottic and posterior glottis stenosis (Fig. 1).
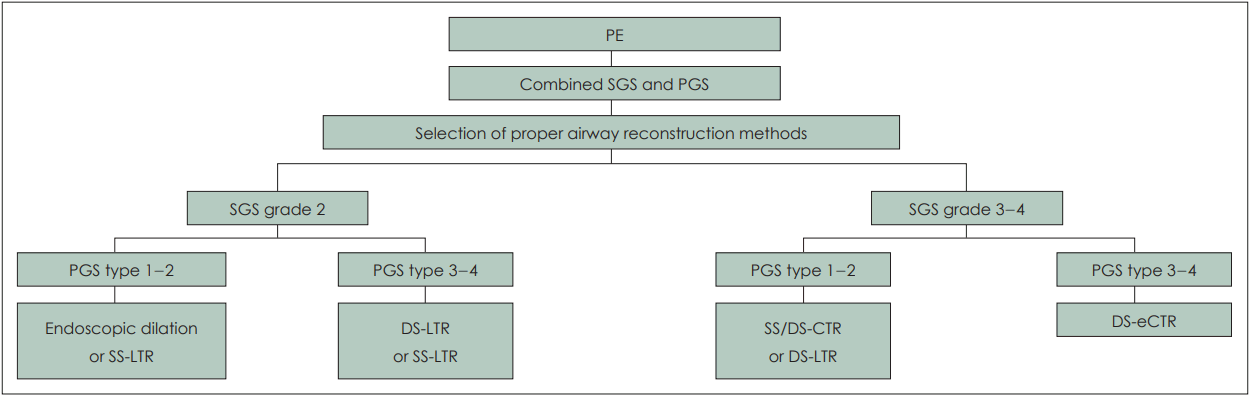
Selection of airway reconstruction methods in children with combined subglottic and posterior glottis stenosis according to PE and grade of airway stenosis. PE, preoperative evaluation; SGS, subglottic stenosis grade; PGS, posterior glottic stenosis; LTR, laryngotracheal reconstruction; SS, single stage surgery; DS, double stage surgery; CTR, cricotracheal resection with end-to-end anastomosis; eCTR, extended CTR.
There were 2 inappropriate PO. A child (case #1) who received apCS with apCG was kept intubated for 7 days at a pediatric ICU as a routine postoperative care of single stage LTR. Fever, thick secretion, and pneumonic infiltration at chest X-ray developed from the 5th postoperative day. The anterior CG was extruded during the evaluation under general anesthesia at postoperative day 7. Although decannulation was maintained for about 14 months since that time, she received an additional aCS with aCG to relieve the dyspnea on exertion. Another child (case #7) developed seizure during postoperative care at the ICU. He received single stage LTR and trachea resection and end-to-end anastomosis. Airway evaluation was done at postoperative day 7 under general anesthesia and extubation was performed. After returning to the ICU, continuous positive airway pressure was applied with facial mask because the chest retraction developed when the child became awake and irritable. Generalized type seizure began at that night with desaturation event, which was changed into left-side localized type seizure and associated with altered mentality for about one month. Emergency tracheostomy was done at the next day of the event. Fortunately, he recovered without neurological sequalae and decannulation was done at 4 months postoperatively without further open airway surgery.
In our series, every child received multiple ancillary endoscopic procedures (mean, 20.6 times; range, 7-42 times) before and after the open reconstruction surgery, which included a rigid endoscopic evaluation under general anesthesia, incision and balloon dilation, removal of granulation tissue, and scar tissue release with mitomycin-C application. Three children (case #2, #3, #4) received laser posterior cordotomy or cordectomy without improvement in the airway but with worsening of PGS. It is important to select the proper candidate for endoscopic management since overenthusiastic endoscopic interventions might worsen the complexity and severity of the stenosis. In a moderate to severe glottic stenosis, laser excision of the posterior glottis should be avoided or carefully applied, especially in children.
All the children in our series had tracheostomy cannulas before their open airway surgery. Suprastomal collapse was another reason of decannulation failure in these children because CS with CG primarily expands the lateral dimension of the cricoid cartilage and upper trachea, which may distort the laryngotracheal framework, resulting in tracheomalacia. The presence of moderate or severe tracheomalacia was an independent factor predicting reintubation in children with LTS [17]. In our study, three children (case #3, #5, #7) with tracheomalacia finally achieved decannulation after CTR, which was performed before, after or concurrently with apCG, respectively. Although CTR is a highly recommended option for severe subglottic stenosis, CTR increases the risk of recurrent laryngeal nerve injury, possible inhibition of laryngeal growth, and dehiscence at anastomosis site [18,19]. One child (case #3) experienced partial disruption of the thyrotracheal anastomosis site, which was managed with a temporary tracheostomy. Theoretically, eCTR may be the most effective reconstructive surgery for a severe multilevel airway stenosis, because it could expand narrow subglottis by CTR and release posterior glottic fixation by LTR with CG [11]. Previous studies presented the success rate of eCTR as 56% [20] and 80% [11] in children with combined airway stenosis. In our series, we could achieve successful decannulation with performing eCTR (case #5) or sequential CTR before/after LTR (case #3, #7).
In conclusion, open reconstructive airway surgery in children is still a challenging issue especially in multilevel complex airway stenosis. To improve the surgical outcomes, precise preoperative dynamic airway evaluation, adequate selection of reconstruction type, appropriate choice of stent and staging, and careful postoperative care were crucial steps.
Acknowledgements
None
Notes
Author Contribution
Conceptualization: Nayeon Choi, Young-Ik Son. Data curation: Nayeon Choi, Jae Hyuk Choi, HeeJung Kim, Yujin Heo, Joo Hyun Park. Formal analysis: Nayeon Choi, Jae Hyuk Choi, HeeJung Kim, Man Ki Chung, Han-Sin Jeong, Chung-Hwan Baek, Young-Ik Son. Investigation: Nayeon Choi, Jae Hyuk Choi, Joo Hyun Park, Man Ki Chung, Han-Sin Jeong, Chung-Hwan Baek, Young-Ik Son. Methodology: all authors. Project administration: Nayeon Choi, Young-Ik Son. Resources: Nayeon Choi, Young-Ik Son. Software: Nayeon Choi, Jae Hyuk Choi, Yujin Heo, Joo Hyun Park. Writing—original draft: Nayeon Choi, HeeJung Kim, Yujin Heo. Writing—review & editing: Man Ki Chung, Han-Sin Jeong, Chung-Hwan Baek, Young-Ik Son.