잘못 시행된 경피적 확장 기관 절개술로 지연된 윤상갑상막절개술에 의한 후두 합병증
Laryngeal Complications of Prolonged Cricothyroidotomy by Misplaced Percutaneous Dilatational Tracheostomy
Article information
Trans Abstract
Despite several advantages, percutaneous dilatational tracheostomy (PDT) performed by physicians who are unfamiliar with head and neck anatomy can result in malpractice and cause significant complications although laryngeal complications due to prolonged cricothyroidotomy caused by malpractice PDT have not been reported. Here we report a 64-year-old female who complained of dysphonia while receiving a fenestrated tube. The tube was mispositioned tube caused by malpractice PDT; the tube was placed at the cricothyroid membrane for four months without awareness. To correct subglottic stenosis and vocal cord edema, the patient underwent tracheostomy conversion and suspension laryngomicrosurgery. Although her voice slightly improved after the operation, she was unable to decannulate the tube and developed laryngeal web and subglottic stenosis. To reduce complications, PDT must be performed with accurate knowledge of anatomy
Introduction
Tracheostomy is generally a technique that creates an alternative airway by opening a window in the tracheal ring. However, cricothyroidotomy is a technique penetrating cricothyroid membrane in emergent situations such as airway obstruction, edema, and trauma [1-3]. Although emergent tracheostomy is sometimes performed, cricothyroidotomy has been reported to have a lower complication rate than emergent tracheostomy in airway emergencies [4]. After a successful cricothyroidotomy, it is recommended to convert to a tracheostomy within 72 hours to prevent laryngeal complications, especially subglottic stenosis. Most studies dealing with cricothyroidotomy focus on immediate results and report fewer long-term complications [4-7].
With the development of percutaneous dilatational tracheostomy (PDT), tracheostomy is performed immediately at the bedside by neurosurgery, pulmonology, and thoracic surgery departments [8]. A systematic review has found that PDT is simpler and easier procedure with reduced rates of infection, scarring, and mortality [9]. However, PDT is not without its own potential complications [10,11]. Inexperienced physicians unfamiliar with head and neck anatomy may place the tube in wrong position or orientation and damage surrounding structures.
Currently, there are no studies detailing the changes that may occur in the larynx due to prolonged cricothyroidotomy and late conversion to tracheostomy.
Herein, the authors would like to introduce the case of a patient with laryngeal complications who maintained cricothyroidotomy for 4 months due to malpractice PDT and underwent late tracheostomy conversion for decannulation and voice improvement.
Case
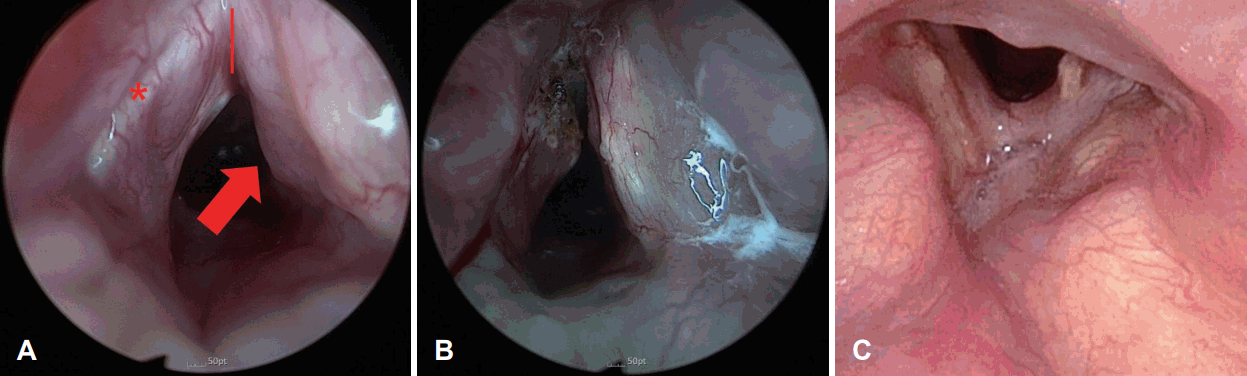
A 64-year-old female hospitalized patient was consulted to the department of otorhinolaryngology with complaint of poor vocalization despite using a fenestrated tube. The patient was treated for traumatic subdural hemorrhage and underwent PDT in the intensive care unit by neurosurgeon because of prolonged intubation at the previous hospital. She was transferred to our hospital for rehabilitation with a tracheostomy tube. Upon physical examination, the tracheostomy tube was positioned high up to the cricoid cartilage, and redness was observed in the surrounding tissues. The tracheostomy tube was observed in the subglottis during the laryngoscopic examination (Fig. 1A). Vocal cord mobility was fixed by the tube, and mucosal waves were not detected. When the CT scan taken at the previous hospital was reviewed, it was confirmed that the tracheostomy tube was located between the cricoid cartilage and thyroid cartilage (Fig. 1B). The patient was diagnosed with tracheostomy tube malposition, meaning that the tube was not in trachea but in cricothyroid membrane. Therefore, the tracheostomy conversion and cricothyroid membrane repair were performed under general anesthesia. Glottic and subglottic edema was observed at right side and suspicious fibrotic scar was observed at left side under a suspension microscope (Fig. 2A). Adhesiolysis was performed through suspension laryngomicrosurgery with a CO2 laser, and 0.1 mL of triamcinolone acetonide (Shin Poong Pharm Co., Seoul, Korea) was injected at both true vocal cords (Fig. 2B). One month after the surgery, the voice and larynx were evaluated after changing to a fenestrated tube. Laryngeal web was observed, but the airway within the larynx has widened (Fig. 2C). As the tube was removed from the subglottis, the movement of both vocal cords partially recovered, but mucosal waves were not detected due to incomplete glottis closure. Only a strained voice due to contact of both false vocal cords was shown. After the operation and rehabilitation, her voice slightly improved. Because neurologic complications, such as seizure, were occurred during the process of decannulation, she was unable to extubate the tube and had regular outpatient follow-ups. Surgeries, such as laryngeal web adhesiolysis and keel insertion are considered to improve the laryngeal complications after general conditions improve.
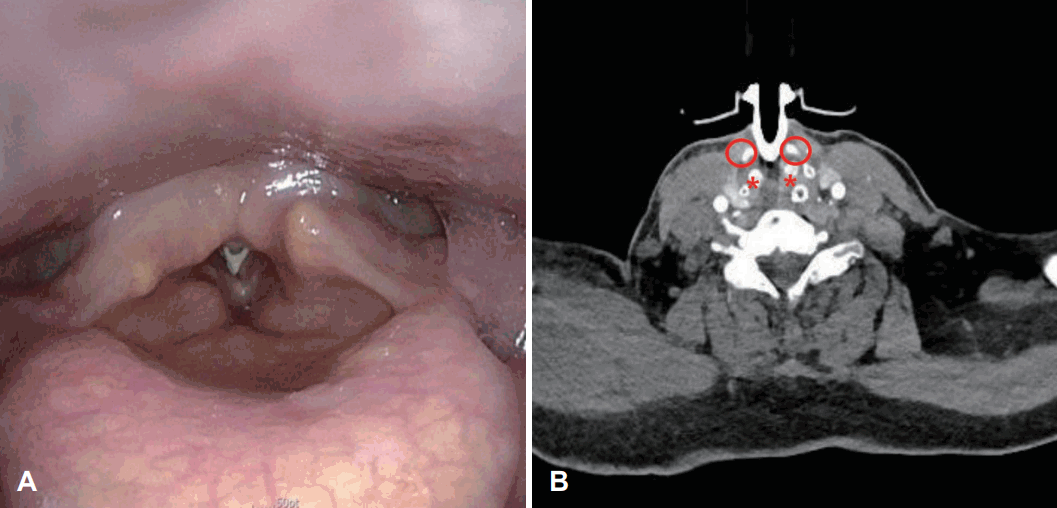
Preoperative findings. The tube was seen below the true vocal cords at the laryngoscopic exam (A), CT scan shows that the tube was placed between the thyroid cartilage and cricoid cartilage (B). Circle indicates the thyroid cartilage. *indicates the cricoid cartilage.
Discussion
A clinical anatomy review of cricothyroidotomy showed the average distance between the cricothyroid membrane and the vocal cord was 9.78 mm [2]. Since the outer diameter of tube in adults is longer than 10 mm generally, vocal cords are likely to be damaged by the tube. Physicians should suspect that the location of the tracheostomy may be incorrect if inflammation at the stoma is repeated. If patients are not able to speak despite the use of a fenestrated tube, they should be referred to otorhinolaryngologists to assess the vocal cords through the flexible laryngoscope. Otorhinolaryngologists need to carefully examine whether the subglottis has stenosis and whether the tube is visible.
The rate of chronic subglottic stenosis among survivors after cricothyroidotomy was 2.2% [5]. Subglottic stenosis occurs due to chronic inflammation induced by repetitive irritation, leading to fibrous scarring [12]. Other reported complications were tracheal stenosis, suprastomal stenosis, voice color change, dysphagia [5].
Although the evidence grade is very low, a systematic review and meta-analysis reported that subglottic stenosis was rare after cricothyroidotomy and that routine conversion from cricothyroidotomy to tracheostomy may not be necessary [7]. Graham, et al. [6] insisted that there was no difference in airway problems after decannulation between the cricothyroidotomy group and the tracheostomy conversion group. However, in Graham’s study, the duration from the procedure to decannulation was 2-7 days, with an average of 4 days in patients who had immediate and successful decannulation without conversion to tracheostomy [6]. Decannulation without tracheostomy conversion would be better if decannulation was expected to be possible within several days. However, it would be advantageous to convert to tracheostomy as soon as possible when the period is expected to be prolonged. It is important to decide whether and when to convert to a tracheostomy considering risks, benefits, and comorbidities. It is necessary to establish precise guidelines through additional research.
A recent review found no difference in mortality or serious adverse events between percutaneous and surgical tracheostomies [9]. However, physicians who are unfamiliar with the head and neck anatomy may commit malpractice. Malpractice may not cause immediate problems, but could lead to long-term complications, such as dysphonia, subglottic web/stenosis, and permanent tube placement. Accurate knowledge of the anatomy around the trachea and meticulous marking of the boundaries through palpation are essential during the procedure. Until physicians become accustomed to PDT, it can be helpful to reduce complication rate by identifying the exact location under guidance by ultrasonography or bronchoscopy [8,13].
Simon, et al. [11] identified hemorrhage, airway complications, and tracheal perforation as the main causes of death following PDT. Zouk, et al. [10] reported late complications of PDT, such as post-tracheostomy tracheal stenosis, trachea-innominate artery fistula, tracheoesophageal fistula, and stomal infection. They emphasized the importance of strict consideration of contraindications such as infection, malignant involvement of anterior neck, hemodynamic instability, high ventilator settings, and non-palpable landmarks. To reduce complications of PDT, bronchoscopic guidance during the procedure, performance by an experienced team, avoidance of a low tracheostomy puncture site, and avoidance of guidewire kinking are important [10,11].
In summary, we reviewed the complications in the larynx caused by prolonged cricothyroidotomy due to a misplaced PDT. To reduce complications, PDT must be performed with accurate knowledge of anatomy.
Acknowledgements
None
Notes
Author contributions
Conceptualization: Sang Jae Lee, Yoon Seok Choi. Data curation: Sang Jae Lee, Yoon Seok Choi. Formal analysis: Si-Youn Song, Hyung Gyun Na. Funding acquisition: Si-Youn Song, Yoon Seok Choi. Investigation: Hyung Gyun Na, Si-Youn Song. Methodology: Sang Jae Lee, Yoon Seok Choi. Project administration: Si-Youn Song, Yoon Seok Choi. Resources: Hyung Gyun Na, Si-Youn Song. Software: Si-Youn Song, Yoon Seok Choi. Supervision: Yoon Seok Choi. Visualization: Sang Jae Lee, Hyung Gyun Na. Writing—original draft: Sang Jae Lee. Writing—review & editing: Sang Jae Lee, Yoon Seok Choi.