제 3병기 설암에서의 단일 림프절 전이 및 보조 요법
Single Lymph Node Metastasis and Adjuvant Therapy in Stage III Tongue Cancer
Article information
Trans Abstract
Background and Objectives
We analyzed treatment results among stage III oral tongue squamous cell carcinoma (OTSCC) patients and performed subgroup analyses according to the depth of invasion (DOI) and node positivity.
Subjects and Method
We retrospectively analyzed the medical records of 87 stage III OTSCC patients.
Results
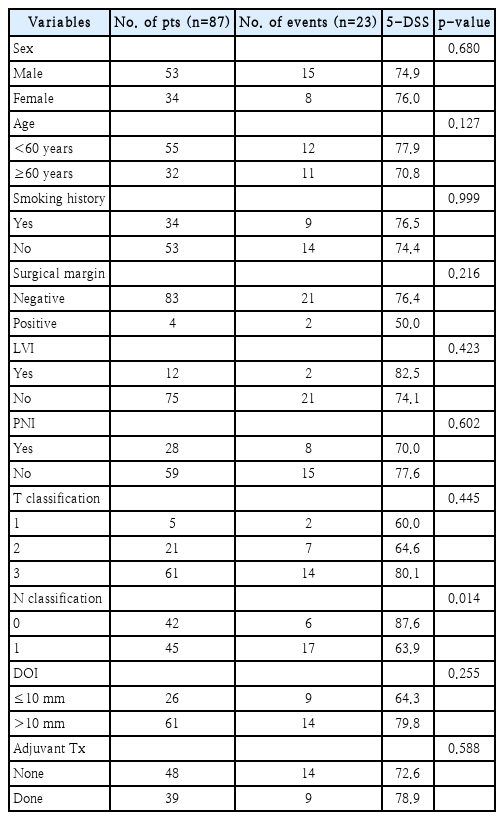
A total of 32 recurrences occurred during the study period, eight cases of local recurrence, 12 cases of regional recurrence, eight cases of distant metastasis, and four cases of loco-regional recurrence. During the study period, 27 patients died due to disease progression. The 5-year recurrence-free survival of the patients was 62.9%, and the 5-year disease-specific survival (DSS) was 75.3%. When prognostic factors related to disease recurrence and survival were analyzed through univariate and multivariate analyses, only the N classification was significantly associated with patient survival (hazard ratio 0.36, confidence interval 0.14-0.91). The 5-year DSS of N0 patients was 87.6%, and the 5-year DSS of N1 patients was 63.9%. Among N1 patients, the 5-year DSS was 90.5% when adjuvant therapy was administered and 63.9% when adjuvant therapy was not administered, which is a statistically significant difference. We did not observe any difference in treatment outcome or survival between patients with superficial (DOI ≤10 mm) or deep lesions (DOI >10 mm).
Conclusion
Among the stage III OTSCC patients, patients with single lymph node (LN) metastasis (N1) showed a distinct prognostic difference from other patients (N0), and the administration of adjuvant therapy to patients having single LN metastasis without other risk factors significantly improved their 5-year DSS.
Introduction
Oral cavity squamous cell carcinoma (OCSCC) is the ninth most common malignancy worldwide [1]. Oral tongue squamous cell carcinoma (OTSCC) is the most common type of OCSCC and has a poorer prognosis than OCSCC in other areas of the oral cavity. The depth of invasion (DOI) is an important prognostic factor related to patient survival, with a higher DOI associated with a higher risk of nodal metastasis, recurrence, and OCSCC-related death [2]. The 7th American Joint Cancer Committee (AJCC) staging system reflected only tumor size and disease extent for the T classification, but DOI is newly included in the revised 8th AJCC staging system [3]. Also, because extranodal extension (ENE) is newly reflected in the N classification of 8th AJCC staging system, cases of metastatic lymph node (LN) with ENE findings have been upstaged to N2a or N3b. Accordingly, all OTSCC patients classified as stage III do not have ENE findings known as a major risk factor. Because patients with a tumor size >4 cm, DOI >10 mm, or N1 are classified as stage III OTSCC, they inevitably have heterogeneity in terms of the composition of patients within the same stage group. Therefore, it is necessary to investigate whether specific subgroups among them show distinctive prognosis difference. In this study, we analyzed the treatment outcomes of stage III OTSCC patients and performed subgroup analysis according to DOI and node positivity. We also analyzed clinical implication of single LN metastasis and adjuvant radiotherapy in these patients.
Subjects and Methods
This study was conducted on patients diagnosed with OTSCC who underwent surgery at Severance Hospital between June 2005 and January 2020. Yonsei University’s Institutional Review Board approved the retrospective study (2020-3627-001) and waiver of informed consent of patients due to it’s retrospective design. The inclusion criteria are as follows: 1) Patients who were histologically diagnosed with OTSCC and underwent surgery and 2) patients with stage III OTSCC (pT1-3N0 or pT1-3N1). The exclusion criteria were as follows: 1) Patients who had previously undergone surgery or radiotherapy in the head or neck area, 2) OTSCC patients with stage I-II or stage IV disease, and 3) patients with distant metastases at the initial visit.
Patient age, sex, smoking history, etc., were analyzed retrospectively from medical records, along with the following pathological information: histologic grade, surgical margin status, lymphovascular invasion (LVI), perineural invasion (PNI), ENE, tumor size, and DOI. During the study period, disease recurrence was evaluated and classified as local, regional, or distant metastasis according to the site of recurrence. The period between the initial treatment and disease recurrence detected in an imaging study or histologic examination was defined as the period of recurrence. Patient mortality was evaluated, and disease-specific survival (DSS) was analyzed by classifying whether death was caused by a disease or something else. Recurrence was defined as a secondary primary tumor when it occurred more than 2 cm away from the index tumor or more than 5 years after the index tumor. The incidence of a second primary lesion was investigated during the study period.
All patients enrolled in this study underwent surgery with therapeutic purposes. Depending on the size and location of the lesion, hemi- or subtotal glossectomy was performed. For cN0 cases, elective neck dissection of levels I-III was performed, and for cN1 cases, therapeutic neck dissection was performed. Adjuvant therapy was considered in the presence of positive/close margin, LVI, PNI, ENE, or T3-4 disease. Adjuvant therapy can reduce the risk of recurrence and disease-related mortality in high-risk patients, but it can be accompanied by treatment-related morbidity. Among patients with a single LN metastasis without major risk factors such as ENE or a positive margin, adjuvant therapy was omitted in selected patients after a sufficient explanation of its risks and benefits.
Clinical information, pathologic information, recurrence, recurrence date, recurrence cause, death, cause of death, date of death, etc. were collected and analyzed. Univariate and multivariate analyses were performed to investigate the prognostic factors affecting recurrence and patient survival. We evaluated the difference in categorical variables between two independent groups using chi-square or Fisher’s exact test. An independent two-sample t-test was used to evaluate the differences in continuous variables between two independent groups. The multivariate Cox proportional hazards regression model has been used to simultaneously evaluate the effects of several factors on distant metastasis and patient survival. The Kaplan-Meier curve was used to analyze disease-free survival, and the survival results were evaluated using a log-rank test. A p-value<0.05 was considered to represent statistical significance. Statistical analyses were performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
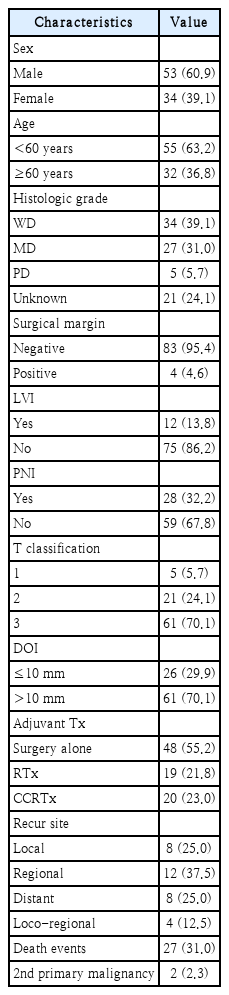
We enrolled 87 patients with stage III OTSCC in this study, of whom 53 (60.9%) were male and 34 (39.1%) were female. The mean age of the patients was 53.2 years (range, 24-75). Tumor staging was evaluated using the 8th AJCC staging system. Out of the total patients, 5 patients (5.7%) were classified as T1, 21 patients (24.1%) as T2, and 61 patients (70.1%) as T3 based on the T classification. Tumor differentiation was assessed, and we found that 34 patients (39.1%) had well-differentiated tumors, 27 patients (31.0%) had moderately differentiated tumors, 5 patients (5.7%) had poorly differentiated tumors, and for 21 patients (24.1%) tumor differentiation was unknown. Surgical margins were assessed, and we found that 83 cases (95.4%) had negative margins, while 4 cases (4.6%) had positive margins. LVI was found in 12 patients (13.8%), and PNI was found in 28 patients (32.2%). DOI was determined, and 26 cases (29.9%) had a DOI of ≤10 mm, while 61 cases (70.1%) had a DOI of >10 mm. Adjuvant therapy was administered to 39 patients (44.8%), with radiotherapy alone given to 19 patients and concurrent chemoradiotherapy given to 20 patients. During the study period, we observed 32 recurrences, including 8 cases of local recurrence, 12 cases of regional recurrence, 8 cases of distant metastasis, and 4 cases of loco-regional recurrence. Disease progression led to the death of 27 patients during the study period. Two patients received additional therapy because a second primary malignant lesion developed in another area of the head or neck during the follow-up period. The 5-year recurrence-free survival (RFS) rate among the patients was 62.9%, and the 5-year DSS rate was 75.3% (Fig. 1). Other clinical information for the patients is summarized in Tables 1 and 2.

The 5-year recurrence-free survival (RFS) of patients was 62.9% (A), and the 5-year disease-specific survival (DSS) was 75.3% (B).
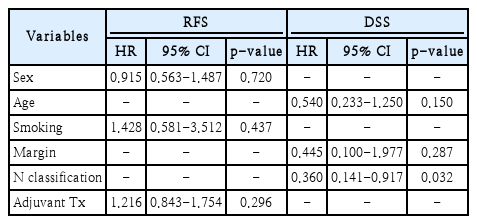
When examining prognostic factors related to disease recurrence, no significant correlation was found in the univariate analyses for any clinical or pathologic factors (Table 3). Additionally, in the multivariate analysis using variables with a p-value of 0.3 or less in the univariate analysis, no significant prognostic factors related to disease recurrence were observed (Table 4). However, when analyzing prognostic factors related to mortality, the N classification showed a statistically significant correlation in the univariate analysis (Table 5). This significant association remained even in the multivariate analysis that included only variables with a p-value of 0.3 or less in the univariate analysis (Table 4).
In survival analyses based on two important prognostic factors - DOI (≤10 mm vs. >10 mm) and N classification (N0 vs. N1) - a statistically significant difference was observed in the 5-year DSS of N0 patients (87.6%) vs. N1 patients (63.9%). However, there was no significant difference in the 5-year DSS of patients with DOI ≤10 mm (64.3%) vs. DOI >10 mm (79.8%) (Fig. 2). To evaluate the effect of adjuvant therapy on treatment outcomes, a subset analysis was conducted on N1 patients without other poor prognostic factors such as LVI, PNI, or ENE. Among these patients, the 5-year DSS was 90.5% when adjuvant therapy was administered and 63.9% when adjuvant therapy was not administered, indicating a statistically significant difference. However, when analyzing the effect of adjuvant therapy on N0 patients without other poor prognostic factors, the 5-year DSS was 100% when adjuvant therapy was administered and 85.7% when adjuvant therapy was not administered, but this difference was not statistically significant (Fig. 3).

The 5-year disease-specific survival (DSS) of N classification and DOI. A: The 5-year DSS of N0 patients was 87.6%, and the 5-year DSS of N1 patients was 63.9%, which was a statistically significant difference. B: The 5-year DSS of patients with depth of invasion (DOI) ≤10 mm was 64.3%, and the 5-year DSS of patients with DOI >10 mm was 79.8%, which was not a significant difference.

The 5-year disease-specific survival (DSS) of N1 and N0 patients with or without Adjuvant therapy. A: Among N1 patients, the 5-year DSS was 90.5% when adjuvant therapy was administered and 63.9% when adjuvant therapy was not administered, which was a statistically significant difference. B: Among N0 patients, the 5-year DSS was 100% when adjuvant therapy was administered and 85.7% when adjuvant therapy was not administered, which was not a significant difference.
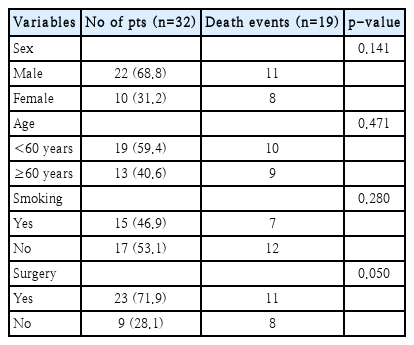
During the study period, 32 relapses occurred, and upon analyzing prognostic factors related to treatment outcomes among relapsed patients in the univariate analysis, only surgical treatment showed a significant correlation with patient survival (Tables 6 and 7). Salvage therapy, which included surgery, was performed in 21 cases. Eight patients underwent chemotherapy or radiation treatment, and the remaining 3 patients did not receive any treatment. The 5-year DSS of relapsed patients after salvage therapy was 42.9% (Fig. 4).
Discussion
The revised 8th edition of the AJCC staging system now incorporates DOI into the T classification for OTSCC. Tumors larger than 4 cm or with a DOI of 10 mm or more are classified as T3, which can represent either superficial (DOI ≤10 mm) or deep (DOI >10 mm) lesions. Histologically, it is well known that oral cancer patients with deep lesions (DOI >10 mm) have a poor prognosis. Therefore, further research among patients with stage III OTSCC is needed to confirm whether those with superficial (DOI ≤10 mm) and deep (DOI >10 mm) lesions show distinct prognostic differences [4,5]. In this study, we compared and analyzed patient DSS based on DOI >10 mm and found no significant differences between the two groups (DOI ≤10 mm vs. DOI >10 mm). In a previous study by Tandon et al., they divided their patient group based on DOI >10 mm and reported a significant difference in recurrence survival, but overall survival did not show any significant difference. However, due to the small number of patients, they did not perform subgroup analyses according to adjuvant therapy, and therefore, we should be cautious when interpreting their results. Typically, T3 and T4 lesions are considered high risk, and adjuvant therapy is recommended. However, it is still unclear whether subgroups by DOI indicate distinctive prognoses among stage III OTSCC patients. Hence, further research is necessary to investigate the clinical implications of DOI and to determine the effect of adjuvant therapy on the survival times of patients with deep lesions (DOI >10 mm).
Because cases with ENE findings are upstaged to N2a or N3b in the N classification of the 8th AJC staging system, not all OTSCC patients corresponding to stage III have ENE findings. We analyzed whether treatment outcomes and patient survival rates differed according to single LN metastasis in the stage III OTSCC patient group without ENE findings, and we confirmed a significant difference in the 5-year DSS between N0 and N1 groups. Traditionally, indications for adjuvant therapy have been an important issue of debate in treating OTSCC patients [6-8]. In particular, there is controversy over whether adjuvant therapy can be omitted in patients with a single LN metastasis without major risk factors such as ENE or a positive margin. In this study, we analyzed the effect of adjuvant radiotherapy on patient survival among patients classified as pN1 using the 8th edition AJCC staging system. We found that when adjuvant therapy was administered to N1 patients without other adverse features, a significant improvement was observed in their 5-year DSS. Chen, et al. [9] also conducted a study of 243 patients with low/intermediate risk pN1 oral cancer and reported no significant difference according to the use of adjuvant therapy. However, in a subgroup analysis conducted among OTSCC patients, they reported that the local and regional RFS of those who received adjuvant therapy was significantly better than that of those who did not receive adjuvant therapy. It is known that OTSCC has a higher loco-regional recurrence rate and lower overall survival rate and cancer-specific survival rate compared to other oral cancers. Therefore, adjuvant therapy is thought to be beneficial in improving OTSCC patient survival given its aggressive features [10-12].
During the study period, there were 32 recurrences, with regional recurrence accounting for 37.5%, local recurrence 25.0%, and distant metastasis 25.0%, and the remaining 12.5% experiencing loco-regional recurrence. None of the clinical pathologic factors were significantly correlated with disease recurrence, and adjuvant therapy after initial treatment did not show a significant correlation with disease recurrence either. Although the main cause of treatment failure was regional recurrence, comparing the disease recurrence patterns between stage III OTSCC patients with N0 and N1 did not show a significant difference in the disease recurrence site.
Salvage surgery was the only statistically significant factor in recurrent patients, and among patients who experienced disease recurrence, the 5-year DSS was only 42.9% after salvage therapy, including surgery, chemotherapy, and radiotherapy. Therefore, the prevention of disease recurrence is crucial for improving the survival rate of stage III OTSCC patients, and efforts should be made to minimize recurrence by setting the optimal extent for surgery and delineating appropriate indications for adjuvant therapy. In particular, Adjuvant therapy should be actively considered in N1 OTSCC patients to improve their survival regardless of other adverse features.
This study has some limitations that need to be considered. Firstly, the study was retrospective, which may have introduced selection bias. Additionally, the lack of statistically significant association between DOI and prognosis in stage III OTSCC patients observed in this study may conflict with previous research on this issue. Furthermore, since this study was conducted with a limited number of patients, further studies with a larger number of patients should be confirmed. Also, regarding the role of adjuvant therapy in single LN metastasis of stage III OTSCC, we noted a significant improvement in disease recurrence after adjuvant radiotherapy; however, the number of patients was too small to draw a concrete conclusion. Therefore, further studies with a larger number of patients are needed to address this issue.
In conclusion, among stage III OTSCC patients, patients with single LN metastasis (N1) showed a distinct prognostic difference from other patients (N0), and the administration of adjuvant therapy to patients having single LN metastasis without other risk factors significantly improved their 5-year DSS.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1C1C1012004).
Notes
Author contributions
Conceptualization: Young Min Park. Data curation: Young Min Park. Formal analysis: Young Min Park. Funding acquisition: Young Min Park. Investigation: Young Min Park. Methodology: Young Min Park. Project administration: Young Min Park. Resources: Young Min Park. Software: Young Min Park. Supervision: Jae-Yol Lim, Yoon Woo Koh, Se-Heon Kim, Eun Chang Choi. Validation: Jae-Yol Lim, Yoon Woo Koh, Se-Heon Kim, Eun Chang Choi. Visualization: Young Min Park. Writing—original draft: Han Cheol Lee, Young Min Park. Writing—review & editing: Han Cheol Lee, Young Min Park.