2000년부터 2019년까지 국내 단일 기관에서 시행된 구강암의 연령 및 성별별 경향성 분석
Age- and Sex-Specific Trends in Oral Cavity Cancer Cancers in a Single Institution From 2000 to 2019 in Korea
Article information
Trans Abstract
Background and Objectives
Head and neck cancer (HNC) represents a significant public health challenge worldwide, impacting mortality rates and economic resources. This study aimed to analyze the age- and sex-specific trends in the prevalence and prognosis of HNCs over two decades.
Subjects and Method
A retrospective analysis was conducted utilizing data collected during the period of 2000 to 2019 from the hospital’s Head and Neck Cancer Center.
Results
We observed that, of the 4167 HNC patients analyzed, the proportion of female with this cancer has remained unchanged over the past two decades. However, the proportion of patients under 40 years of age with oral cavity cancer has significantly increased. We observed a significant improvement in the 3-year overall survival (OS) in HNCs during 2010-2019 compared to 2000-2009. This improvement was particularly pronounced in male, whereas no significant change was observed in female. Notably, the 3-year OS has significantly improved over the past decade for advanced-stage (III & IV) patients.
Conclusion
The incidence of oral cavity cancer has increased among patients under 40 years of age over the past two decades. Additionally, the prognosis of oral cavity cancer patients notably improved, particularly in male under 40 years of age.
Introduction
The incidence of head and neck cancers (HNCs) has been increasing worldwide, with approximately 660000 new cases and 325000 deaths reported annually [1]. The leading cause of HNCs is tobacco use, followed by alcohol consumption, betel quid, and Epstein-Barr virus [2]. However, recent trends have shown a shift towards a higher incidence of human papillomavirus (HPV)-associated HNCs, particularly oropharyngeal cancers, while tobacco-related HNCs are declining [3]. As reported in some studies, the changes in the pathophysiology of HNCs may have contributed to the shift towards a younger age at diagnosis [4].
The prognosis of HNCs has undergone significant changes. The overall survival (OS) rates for HNCs have improved over the years due to advancements in diagnostic techniques, surgical procedures, radiation therapy, and targeted immunotherapy [5,6]. In particular, de-escalating treatment protocols for HPV-associated oropharyngeal cancer have resulted in a dramatic improvement in functional outcomes and quality of life while maintaining oncological outcomes [7]. However, the prognosis for advanced-stage HNCs, particularly those with extracapsular extension or metastatic disease, remains unchanged [2].
HNC profoundly affects patient quality of life and results in considerable healthcare costs, which has significant implications for the social health burden. Therefore, it is essential to review the current epidemiological trends in HNCs to allocate healthcare resources efficiently and develop effective strategies for prevention, early detection, and treatment of the disease. This study reviews the current trends in the epidemiology and prognosis of HNCs, stratified by age and sex at a single institution in Korea.
Subjects and Methods
We conducted a retrospective review of the medical records from Seoul St. Mary’s Hospital between January 2000 and December 2019 to investigate HNC diagnoses. We obtained the database from the hospital’s Head and Neck Center operation team. The database included patient information, such as age, sex, primary site, death status, date of death, treatment modalities, TNM classification, and stage at diagnosis. We compared the prognostic trend by dividing the study period into 2000-2009 and 2010-2019. The HNCs included various histopathological tumor types, such as squamous cell carcinoma, and were staged according to the 7th and 8th editions of the AJCC cancer staging system. As our clinic operates an inpatient hospice, many patients are referred to our hospital for hospice care after being diagnosed and treated for cancer at other hospitals. Therefore, patients who received palliative medicine at our hospital during their initial diagnosis were excluded from the study.
HNCs were classified using the 10th revision of the International Classification of Diseases as follows: oral cavity (C000, C001, C009, C021, C029, C031, C039, C041, C049, C050, C060, C062, C069), salivary gland (C079, C080, C081, C089), oropharynx (C019, C051, C052, C059, C090, C091, C099, C103, C104, C109), nasopharynx (C110, C111, C112, C113, C118, C119), hypopharynx (C129, C131, C132, C139, C140), and larynx (C320, C321, C322, C323, C328, C329). When the tumor extended across two or more sites, we defined the primary tumor location as the area that had the greatest extent of involvement.
Statistical analyses were performed using R software (version 4.0.2; The R Foundation for Statistical Computing, Vienna, Austria). The Spearman’s rank correlation coefficient, often denoted by the Greek letter rho (ρ), is a measure of the strength and direction of the linear correlation between two variables. It is used to analyze correlation between variables that are not normally distributed. The coefficient ranges from -1 to 1, where 0 indicates no correlation. Generally, a coefficient with an absolute value of 0.4 or higher is considered moderate, and 0.6 or higher is considered strong. We used Spearman’s rank correlation coefficient to analyze the linear relationship between patient age/sex and prevalence. The logrank test and Kaplan-Meier curves were used to compare 3-year OS and Cox proportional hazard regression analysis was used to calculate relative risk. A p<0.05 was considered significant for the independent samples t-test.
The Institutional Review Board of our hospital approved this study (KC23RASI0339). The study was conducted in accordance with the relevant laws and regulations, good clinical practices, and ethical principles, as described in the Declaration of Helsinki.
Results
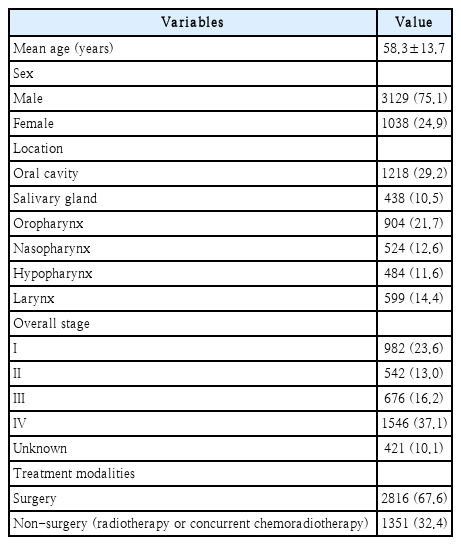
The basic demographics of the HNC patients are presented in Table 1. We enrolled 4167 subjects, with a mean age of 58.3± 13.7 years. The number of male was 3127 (75.1%), which was higher than that of female (1038, 24.9%). The most common primary site of HNC was the oral cavity (1218, 29.2%), followed by the oropharynx (904, 21.7%), larynx (599, 14.4%), nasopharynx (524, 12.6%), hypopharynx (484, 11.6%), and salivary glands (438, 10.5%). The most common stage was stage IV with 1546 (37.1%) patients, followed by 982 (23.6%) patients in stage I, 676 (16.2%) patients in stage III, and 542 (13.0%) patients in stage II. The stage was not recorded for 421 (10.1%) patients. A total of 2816 (67.6%) patients underwent surgery, while 1351 (32.4%) received nonsurgical treatment, such as radiotherapy or concurrent chemoradiotherapy, as initial treatment modalities.
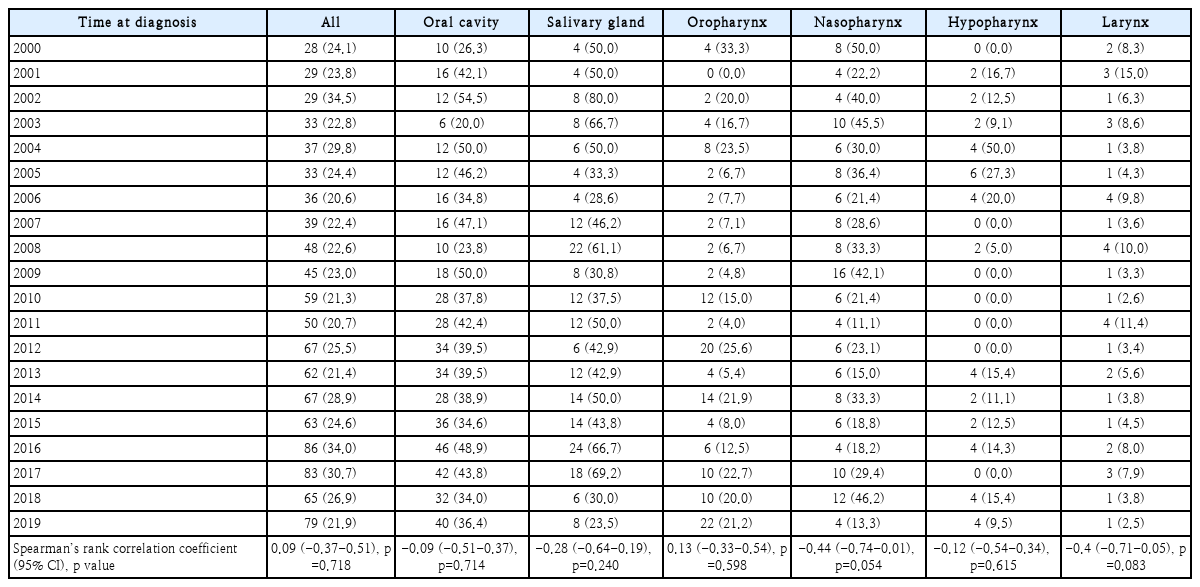
Table 2 shows the proportion of female with HNC based on the location of the primary tumor. The nasopharynx and larynx tended to be moderately negatively correlated with the proportion of female but the relationship was not significant (ρ=-0.44 and -0.40, respectively). The proportion of female in the head and neck subsites remained relatively stable, with no significant change detected even when all such subsites were integrated for analysis (p=0.718).
Table 3 shows the proportion of patients less than 40 years of age with HNC, by primary tumor location. The proportion of patients increased over time in those with cancer of the oral cavity, oropharynx, and nasopharynx (ρ=0.47, 0.18, and 0.14, respectively). Notably, we found a moderate positive correlation between patient age and oral cavity cancer, indicating that patients under 40 years of age were more likely to have oral cavity cancer (p=0.036). The proportions of salivary gland, hypopharynx, and larynx cancers tended to decrease over time, but the results were not significant. Fig. 1 shows the increase in the proportion of young patients with oral cavity cancer over time.
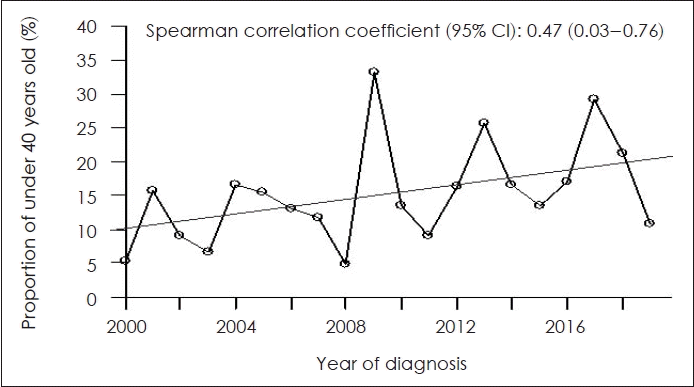
Proportion of patients with oral cavity cancer and age less than 40 years according to the time of diagnosis. CI, confidence interval.
We conducted two separate analyses to evaluate the prognoses of HNC patients under 40 years of age by sex. The first was conducted for all HNC locations, while the second focused particularly on oral cavity cancer. The study period was divided into 2000-2009 and 2010-2019. The results of both analyses are summarized in Table 4. There were no significant differences in 3-year OS for HNC patients as a whole between the two study periods. However, when we focused specifically on oral cavity cancer, there was a significant improvement in 3-year OS over the past decade (63.64% vs. 77.01%, hazard ratio [HR]=0.55, 95% confidence interval [CI]=0.33-0.91, p=0.020). This improvement was particularly pronounced in male (28.57% vs. 72.11%, HR=0.31, 95% CI=0.15-0.64, p=0.002).
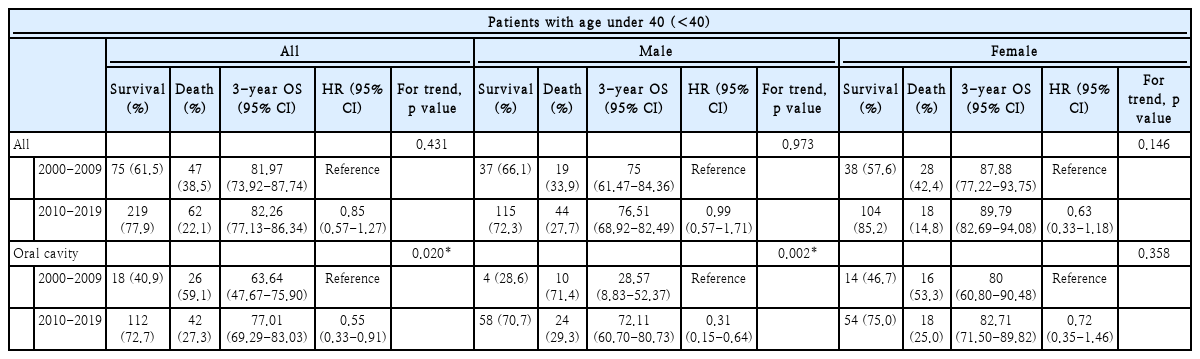
Sex-based analysis of prognostic trends in oral cavity cancer patients less than 40 years of age: comparing 3-year OS between two equal study periods
The study periods were divided into two equal intervals, and the analysis was conducted separately for early-stage (I & II) and advanced-stage (III & IV) patients (Table 5). The analysis detected no significant differences in 3-year OS for early-stage (I & II) patients between the two study periods. However, for advanced-stage (III & IV) patients, OS significantly improved during 2010-2019 compared to 2000-2009 (0% vs. 52.50%, HR=0.31, 95% CI=0.13-0.71, p=0.006).
Discussion
The prevalence of oral cavity cancer has been increasing in young adults worldwide, including in Korea, particularly with regard to oral tongue cance [8,9]. However, the factors responsible for driving this alarming increase in oral cavity cancer incidence remain largely unknown. Several hypotheses have been proposed to explain these rising trends, including changes in dietary patterns, exposure to environmental toxins, chronic trauma (such as dental injury due to ill-fittting dentures), the potential impact of endocrine disrupting agents like bisphenol A, and other unidentified factors [10-12]. Further research is required to fully comprehend the underlying causes of the escalating incidence of oral cavity cancer, particularly in young individuals.
Although the significance of HPV in oropharyngeal squamous cell carcinoma is widely recognized, its potential impact on oral cavity cancers is still not fully understood [13]. Considering the fact that the majority of oral HPV infections tend to resolve within a year, the long-lasting presence of the virus may significantly contribute to the development of diseases associated with HPV [14]. Research has provided evidence that HPV infection can affect gingival tissue, particularly with the periodontal pocket serving as the primary site where basal cells are exposed to environmental factors, potentially leading to oral malignant transformation [15]. The presence of chronic inflammatory processes in periodontitis contributes to the enlargement of the periodontal pocket, thereby promoting increased proliferation of basal cells [16]. This persistent inflammation further contributes to a higher viral load in saliva and an increased risk of HPV transmission [17]. This findings highlights the need for age-specific preventive measures and screening programs. Since 2016, Korea has implemented the HPV vaccine as part of its national mandatory vaccination program for female teenagers aged 12 to 17. The program provides Servaryx or Gardasil for free. This approach has helped prevent individuals from developing unexpected illnesses and has reduced the burden on the public healthcare system. We expect that the implementation of preventive vaccination programs and lifestyle modifications will reduce the prevalence of oral cavity cancer in the younger population in Korea.
In our study, the 3-year OS for oral cavity cancer in young men significantly improved over time, although there was no difference for young female. Although the number of male with oral cavity cancer under the age of 40 years was insufficient to validate our study, we speculate that the improved prognosis may be linked to lifestyle changes. Numerous studies have reported that quitting smoking after a HNC diagnosis improves the prognosis [18,19]. A non-smoking project for public purposes has been widely supported in Korea over the past few decades. As a result, male who had continued to smoke after an HNC diagnosis were motivated to quit smoking, and the treatment outcomes were better in male than female. In contrast, the 5-year OS for oral cavity cancer in male has been constant over time, while the survival rate in female has dramatically improved in China [20]. Contrary to the decrease in the smoking rates observed in many other countries, China has experienced an increase in smoking prevalence [21]. Thus, the aforementioned trend may be interpreted as a manifestation of this situation.
The 3-year OS for oral cavity cancer in male under the age of 40 years significantly increased during 2010-2019 compared to 2000-2009. To clarify the cause of this difference, we analyzed the two study periods by dividing them into two categories: stage I/II corresponding to the early stage, and stage III/IV corresponding to the advanced stage. No differences in prognosis were detected between the two groups in the early stage, however, the 3-year OS improved in patients in the advanced stage during 2010-2019 compared to 2000-2009. This improvement was attributed to advancements in treatment modalities, including the introduction of patient-tailored treatments, hyperfractionated and accelerated radiotherapy, and immunotherapy [20,22,23]. In Pulte and Brenner [24], the 5-year OS of oral cavity cancer, particularly that of the tongue, improved more in patients with the locally advanced stage compared to the early stage between the late 20th and early 21st centuries; our results are consistent with those findings. All patients with advanced-stage oral cavity cancer died during 2000-2009, resulting in a 3-year OS rate of zero. Further medical chart review revealed that the patients during this period had underlying hematologic malignancies, such as Fanconi’s disease and chronic myeloid leukemia, and had undergone bone marrow transplantation, a known risk factor for a poor prognosis [25]. We considered that these comorbidities may have affected our results. However, although there has been an improvement in the survival rate of advancedstage oral cavity cancer over the past decade, this has not translated into an overall improvement in the prognoses of HNCs. This was attributed to the heterogeneous characteristics of HNCs, such as differences in tumor biology, genetic predisposition, and patient demographics, which hindered the overall improvement in the HNC prognosis.
Our study had some limitations. First, it was conducted retrospectively in a single institution, so some selection bias and confounding factors may limit the generalizability of our analysis. Second, the database did not include information on patient lifestyle factors, such as smoking and alcohol consumption, which are known traditional risk factors for HNCs. This served as a limiting factor when interpreting the impact of risk factors on changes in oral cavity cancer trends. Third, our study lacked consistency in the stage distribution analysis due to the new update of the TNM classification during the study period. Fourth, we did not consider the presence of double primary cancers, such as hematological malignancies or lung cancers. Lastly, we did not perform an analysis of the impact of advanced treatment modalities on prognosis improvement in stage III/IV oral cavity cancer. Therefore, we were unable to determine the factors contributing to the improved prognosis observed in advanced-stage oral cavity cancer. Nevertheless, our study is one of the first to evaluate the age- and sex-specific trends in the prevalence and prognosis of HNCs. Our findings highlight the need for targeted preventive measures and screening programs and emphasize the importance of continued research to improve the treatment options for patients with advanced-stage oral cavity cancers and to develop effective strategies for the prevention and early detection of the disease. We anticipate that our study will contribute to reducing the social burden on public health and facilitating the efficient allocation of public health resources.
Acknowledgements
Statistical consultation was supported by the Department of Biostatistics of the Catholic Research Coordinating Center.
Notes
Author Contribution
Conceptualization: Sang-Yeon Kim, Dong-Il Sun. Data curation: Jung-Hae Park, Oh-Hyeong Lee, Geun-Jeon Kim. Formal analysis: Jooin Bang. Investigation: Jung-Hae Park, Oh-Hyeong Lee, Jooin Bang, Geun-Jeon Kim. Methodology: Jung-Hae Park, Oh-Hyeong Lee, Jooin Bang, Geun-Jeon Kim. Supervision: Sang-yeon Kim, Dong-Il Sun. Writing—original draft: Jooin Bang. Writing—review & editing: Jooin Bang, Sang-yeon Kim, Dong-Il Sun.