상악동에서 발생한 점액섬유육종 1예
A Case of Myxofibrosarcoma in the Maxillary Sinus
Article information
Trans Abstract
Myxofibrosarcoma (MFS) is a rare subtype of soft tissue sarcoma, most commonly occurring in the lower extremities and rarely in the head and neck region. To our knowledge, there have been no reported cases of MFS originating from the maxillary sinus in Korea. Herein, we describe a case of a 61-year-old male who presented with left-sided hypoesthesia and a mass in the maxillary sinus, which was subsequently diagnosed as MFS originating from the maxillary sinus through endoscopic surgery with a prelacrimal approach. The patient was administered postoperative radiation therapy, and after two years of follow-up, there was no evidence of recurrence. This report presents a case of successful treatment of MFS in the maxillary sinus.
Introduction
Myxofibrosarcoma (MFS) is a malignant pleomorphic fibroblastic sarcoma, which is a rare histologic subtype of soft tissue sarcoma originating from fibroblasts [1,2]. It is characterized by a distinct curvilinear vascular pattern and a variable myxoid stroma [2]. MFS is observed in a wide age range from adolescence to old age, with the highest incidence detected in people in their 70s [3,4]. It most commonly occurs in the lower extremities (77%), followed by the trunk (12%) and retroperitoneum/mediastinum (8%), but it rarely occurs in head and neck lesions [5,6]. Although the maxillofacial area is not frequently affected, the hypopharynx, sinuses, and parotid glands are most frequently affected [3].
Since the first report of MFS in the maxillary sinus in 1982 [7], only 17 cases have been reported in nine studies outside Korea. In Korea, three cases of MFS originating from the false vocal cord, mandible, and cheek in head and neck lesions have been reported. To the best of our knowledge, there has been no report of MFS originating from the maxillary sinus, making the present case the first to be reported. Hence, this report introduces MFS originating from the maxillary sinus.
Case
A 61-year-old male presented with a 3-month history of left-sided hypoesthesia involving the left cheek, lip, and gum. After consultation with the neurology department and subsequent brain MRI, a mass lesion was identified in the left maxillary sinus. He was referred to the otolaryngology department for further management. He had no underlying disease and was an ex-smoker with a 10-pack-year smoking history. Nasopharyngoscopy revealed no significant findings, except for left-sided septal deviation and yellow, thick discharge in the left middle meatus. A CT scan disclosed a 3.6-cm heterogeneously infiltrative enhancing mass with destruction of the maxillary anterior wall and orbital floor. The mass extended to the zygoma and zygomatic arch, and it was also involving the left middle/inferior turbinate (Fig. 1). The brain MRI demonstrated a partially enhancing mass lesion in the left maxillary sinus, which involved the zygomatic arch and left nasal cavity. The T1-weighted images revealed a low signal intensity, whereas the T2-weighted images revealed a high signal intensity (Fig. 2).
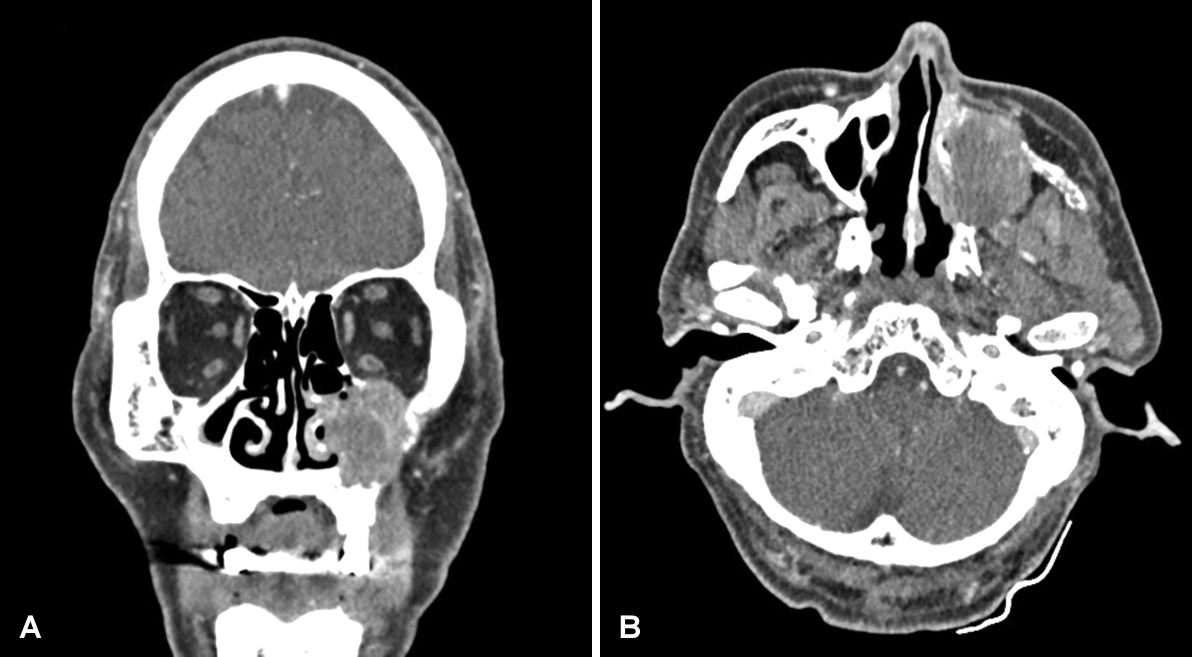
Paranasal sinus CT. An approximately 3.6-cm-sized infiltrative enhancing mass with maxillary anterior wall and orbital floor destructive, which has invaded the left middle/inferior turbinate, left zygoma, left zygomatic arch (A, coronal view; B, axial view).

Brain MRI. Tumor arising from the left maxillary sinus with extensive involvement of the zygomatic arch and left nasal cavity. Gadolinium-enhanced fat-suppressed T1-weighted images demonstrating a distinct radiographic pattern, characterized by low signal intensity and partial enhancement (A, coronal view; B, axial view). T2-weighted images showing a high signal intensity (C).
Endoscopic surgery was performed with a prelacrimal approach to diagnose and remove the mass in the left maxillary sinus under general anesthesia. The mass protruded to the anterior part of the left maxillary sinus ostium. Ocular injury could not be avoided to have sufficient safety margin considering the findings suspicious for malignancy observed in the imaging studies. However, malignancy could not be determined in frozen tissues, so only the gross tumor was removed to the maximum possible extent. Although CT or MRI findings demonstrated tumor invasion in the maxillary sinus mucosa, the mucosa and mass were separated relatively well, and most of the gross mass was removed. The resected mass appeared as two fragmented pieces, measuring 2 and 2.8 cm, respectively, and exhibited a solid and hard consistency, with a lobulated shape and an irregular surface (Fig. 3).

Excised mass. The specimen was fragmented into two pieces during excision, measuring (A) 2 cm and (B and C) 2.8 cm in size, respectively. The lesion appeared lobulated with an irregular surface and was white-to-gray in color with areas of focal hemorrhage.
The histopathologic examination revealed atypical spindle cell sarcoma consistent with MFS of intermediate grade (Fig. 4). The postoperative positron emission tomography-CT (PET-CT) scan demonstrates no residual tumor and absence of any discernible hypermetabolic lesions indicative of lymph node involvement or distant metastasis. Although no prominent remnant tumor findings were observed in the postoperative PET-CT scan, we planned radiotherapy because the pathologic result was a malignant tumor and a sufficient safety margin was not secured during surgery. The patient received 30 sessions of radiation therapy (RTx) with 69 Gy and has been in follow-up for 2 years without evidence of recurrence.
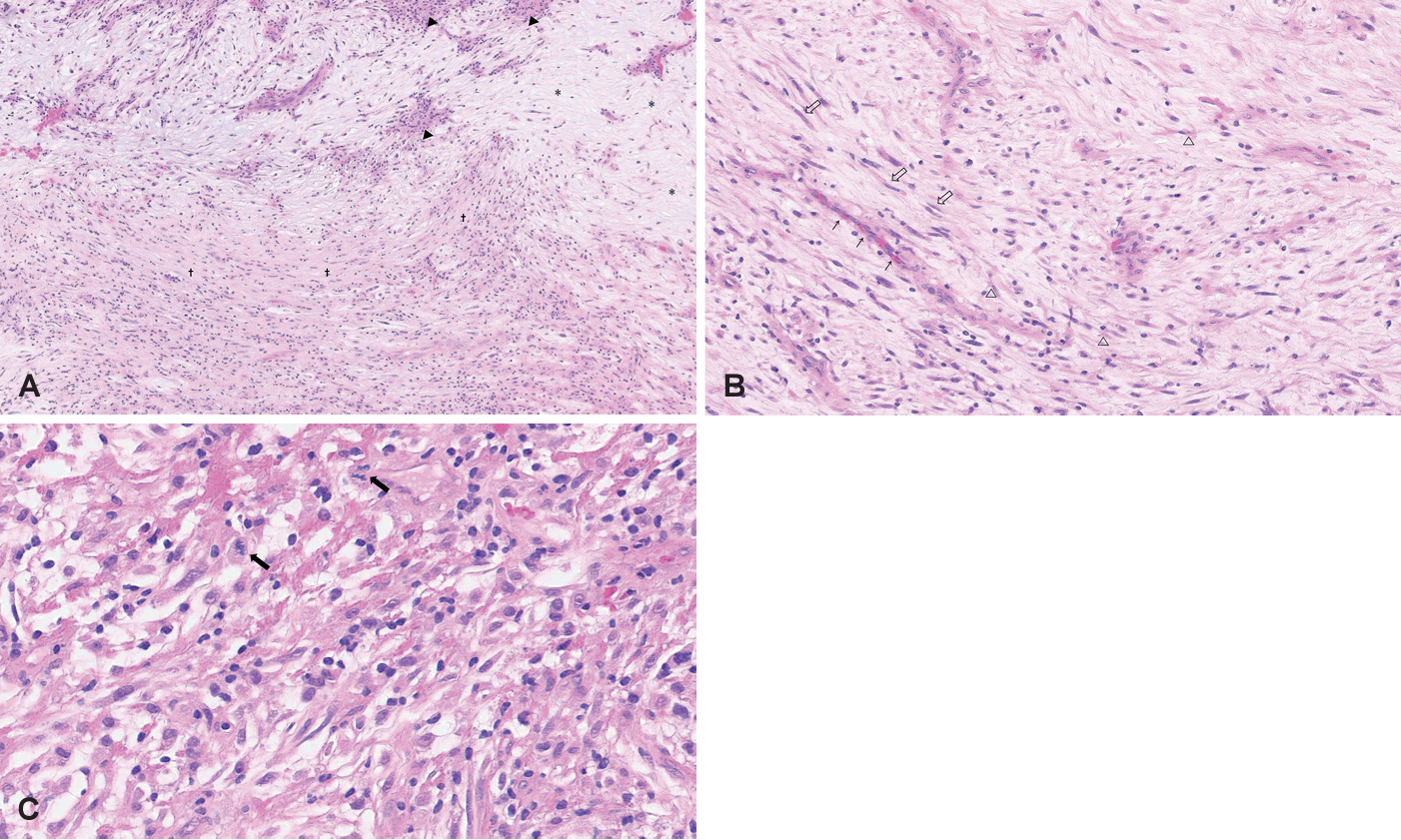
Histopathology (hematoxylin and eosin staining). A: There are hypercellular area (arrowheads), hypocellular area (†), and a myxoid area (*) present in a mixed pattern (×100). B: Curvilinear and thin-walled vessels (thin arrow) are observed within the myxoid matrix, along with the presence of spindle cells (empty arrow) and stellate cells (empty arrowhead) (×200). C: The presence of pleomorphic nuclei is evident and concurrent mitotic activity (thick arrow) is observed (×400).
Discussion
MFS is a fibroblast-derived soft tissue sarcoma commonly found in the extremities and trunk but rarely observed in the head and neck region [1]. It predominantly manifests in the middle-aged and elderly population, with an almost equal incidence rate between male and female [8]. The clinical manifestation of MFS in the sinonasal tract resembles that of other soft tissue neoplasms, which includes symptoms such as epistaxis, nasal obstruction, rhinorrhea, and nonspecific nasal discomfort [1,8]. Although MRI and CT scans can disclose malignant characteristics such as local invasion and aid in diagnosis, they are not conclusive diagnostic tools [1]. CT scans generally reveal bony alterations and either expansive or destructive changes [3], but low-grade MFS can appear as a low-intensity area on CT scans [9]. MRI can demonstrate the complete extent of cancerous tumors more clearly [6]. MFS exhibits hypointense signals on T1-weighted images and markedly hyperintense signals on T2-weighted images. Even in tumors with a focal growth pattern, the infiltrative growth pattern may be noticeable on gadolinium-enhanced fat-suppressed T1-weighted images [8,9]. It is difficult to diagnose MFS in the sinonasal region before receiving a pathological confirmation because of the nonspecific nature of radiographic findings and the absence of distinctive symptoms [6].
MFS displays specific histopathologic features, including alternating areas of hypocellular and hypercellular fibrous tissue, pleomorphic nuclei, curvilinear thin-walled blood vessels in myxoid areas, and the presence of spindle and stellate cells [1,10,11]. The immunohistochemical staining revealed positivity for vimentin and CD-34, indicating a fibroblastic origin of the tumor. Smooth muscle actin was occasionally positive, whereas S-100 protein was negative [1,3,10]. Scattered CD68-positive monocytes/macrophages were also identified [8].
In contrast to various other soft tissue sarcomas, MFS is distinguished by its notable propensity for heightened local recurrence rates, which range between 16% and 61%. This is in comparison to approximately 10% recurrence rates observed in other subtypes of soft tissue sarcoma [12]. The reported rates of distant metastases in the literature vary between 15% and 38% [12]. Following metastasis, there are currently no treatment strategies specific to MFS, and the prognosis is unfavorable [13].
Surgery is the primary treatment for MFS [6]. A wide surgical margin during tumor resection is critical for disease cure [1,8,14], which can be achieved by considering both functional and cosmetic factors. Nonetheless, complete resection with a wide margin is often impossible in the head and neck region [1,8,14] RTx has been proposed as a definitive treatment for unresectable MFS. However, there have been no randomized controlled trials specifically assessing this approach [15]. Adjuvant RTx can be used where the tumor is unresectable or large, the margin after resection is close to within 1 cm, the margin is positive or the tumor is histologically high-grade [1,6,14]. In the present case, although the frozen biopsy during surgery did not indicate malignancy, the tumor was removed to the maximum possible extent because of suspicious findings on imaging studies. The final diagnosis after surgery confirmed the presence of MFS. Nevertheless, due to the proximity of the tumor to the eye and the potential for ocular complications, additional resection for safety margin was not feasible. Moreover, the histopathologic examination showed an unknown margin state, intermediate grade, for which postoperative RTx was considered more appropriate than additional wide excision. The patient has been under surveillance for a period of 2 years, during which no signs of recurrence have been detected.
This case report emphasizes the successful management of MFS originating from the maxillary sinus, and it is the first case reported in Korea. We hope that this case contributes to the understanding of effective treatment for this rare type of soft tissue sarcoma.
Acknowledgements
None
Notes
Author contributions
Conceptualization: Sung Jae Heo. Data curation: all authors. Formal analysis: all authors. Methodology: all authors. Supervision: Sung Jae Heo. Validation: Sung Jae Heo. Writing—original draft: Eun Jung Oh. Writing—review & editing: Sung Jae Heo.
