Robot-Assisted Free Flap Reconstruction for Oropharyngeal Cancer: A Systematic Review
Article information
Abstract
Management of oropharyngeal cancer is undergoing a shift due to different profiles of patients, who tend to be younger and show better prognosis. Transoral robotic surgery is being established as the first-line approach as the procedure has shown minimal invasiveness and good oncological and functional outcomes, while avoiding extensive open surgery approaches and intensive chemoradiotherapy treatments. Naturally, the next step in the management of oropharyngeal cancer would be using the same technology for reconstruction to preserve the function and anatomy of the oropharynx. Free flaps, especially the radial forearm free flap and the anterolateral thigh flap, proved to be ideal for oropharyngeal reconstruction, and robotic assistance in these cases is yet to be explored. A limited number of studies have reported robot-assisted oropharyngeal reconstruction, so this review summarizes the currently available data and explores the feasibility, safety, and functional outcomes of this novel technique.
Introduction
Oropharyngeal squamous cell cancer (OPSCC) is the commonest form of head and neck cancer with 98412 new cases reported globally in 2020 [1]. With changing aetiology due to human papillomavirus (HPV)-association, the incidence and demography is rapidly changing. HPV related OPSCC are known to have a better outcome and an increased number of patients is left to deal with long term comorbidities of intense treatment to control the disease.
Traditionally radiotherapy (RT) +/- chemotherapy is considered the appropriate curative treatment option for patients diagnosed with early-stage OPSCC while open surgery and adjuvant RT +/- chemotherapy as curative treatment for advanced stage locoregional disease [2]. Chemoradiotherapy (CRT) gained popularity in the past, to preserve functions of speech and swallowing by avoiding surgical disruption of the region [3]. Inevitably, these therapies, have also been linked with late complications that impact the quality of life of patients.
On the other hand, surgery aims for tumour resection with clear margins and reconstruction of the affected site when needed. Access to the tumour site is achieved through transmandibular approaches, mandibular lingual release and transpharyngeal approaches [4]. These techniques are accompanied by increased morbidity associated with the invasiveness of these extensive operations. The series of complications following open surgery approaches can be intraoperative and result in nerve injuries and unintentional fractures or postoperative and include facial midline scars, lip deformities, malocclusion, orocutaneous fistula formation and osteoradionecrosis [5,6]. Importantly, functional morbidity regarding speech and swallowing problems should be considered [7].
Transoral robotic surgery (TORS) for head and neck surgery was only recently approved by the US Food and Drug Administration, in 2009 for early-stage oropharyngeal cancer based on the outcomes of a multicentre study of Weinstein and O’Malley which assessed the safety and negative margin tumour resection of TORS, for patients with oropharyngeal and laryngeal cancer [8,9]. Since then, TORS has increasingly gained popularity as s first-line approach for managing early stages of oropharyngeal carcinomas. TORS offers high-resolution visualisation of oropharyngeal anatomy as well as precise movements of the articulating robotics arms. Functional and oncological outcomes of various published retrospective studies show at least comparable or favourable results for patients receiving alternative treatments. Recent systematic reviews evaluating TORS and intensity-modulated RT reported similar survival rates, oncologic and functional outcomes about the two treatment modalities, underlying, however, the lack of randomized controlled trials [10,11].
Since the use of robot assistance in free flap reconstruction for oropharyngeal cancers is still in infancy, the available literature is very patchy. Therefore, the aim of this systematic review is to compile available literature on robot assisted free flap reconstruction for oropharyngeal cancer and present an unbiased update on the topic.
Methods
Search strategy
Original articles were identified for evaluating the use of robotic surgery in free flap reconstruction of oropharyngeal cancer by a comprehensive search of four databases (10 May 2021): PubMed, Web of Science, Ovid Embase and Cochrane Library. There were no initial restrictions on the date of publication or the type of studies. The following free text terms were used to identify articles regarding robot-assisted free flap reconstruction: “robot” OR “TORS” OR “transoral robotic surgery” OR “da Vinci robot” AND “free flap reconstruction” OR “flap reconstruction” OR “reconstruction.” Concerning the regarding robot-assisted free flap reconstruction: “robot” OR “TORS” OR “transoral robotic surgery” OR “da Vinci robot” AND “free flap reconstruction” OR “flap reconstruction” OR “reconstruction.” The total number of articles identified using the above search terms for searching these databases was 716. Following the removal of duplicate publications, we were left with 178 studies. The initial screening of titles and abstracts yielded 53 studies. Finally, after a detailed review of the studies and application of specific inclusion and exclusion criteria, which are described in the next section, 12 studies were selected. A comprehensive review using the PRISMA flowing diagram has been generated and included in the review (Fig. 1).
Inclusion and exclusion criteria
The first stage of study selection was the title and abstract screening in order to narrow down potential studies. Final study selection was made after a full-text review of the articles with the following inclusion criteria in mind: 1) studies in which robotic surgery was performed for patients primarily with oropharyngeal cancer, 2) studies including patients who received free-flap reconstruction 3) published articles: randomised controlled trials, cohort studies, case series and case reports. Exclusion criteria included: 1) preclinical studies, 2) animal studies, 3) non-English papers 4) review studies and 5) studies in which TORS was not used for oropharyngeal cancer 6) studies that did not include flap reconstruction after oropharyngeal tumour removal and 7) studies that reported insufficient data.
Methodological quality
Joanna Briggs Institute Critical Appraisal Checklist for Case reports and case series were used to assess methodological quality and potential bias in the design of the studies. Following the study selection, after considering inclusion and exclusion criteria, no study was subsequently excluded as a result of Joanna Briggs Institute’s Critical Appraisal Checklist, and study data could be used for the synthesis of the results of our systematic review.
Data analysis
Out of the 12 studies that were included in this review, data being recorded included: the year of publication, type of study, the age and sex of patients being treated with TORS, the tumour site as well as the T stage of the oropharyngeal carcinoma. Operation time, follow up time, complications, hospital stay and free flap selection for robotically reconstructing the oropharynx were also recorded. Different measures and scores of the studies for calculating functional outcomes of reconstruction and quality of life have been noted, although not directly comparable.
Results
Population and study characteristics
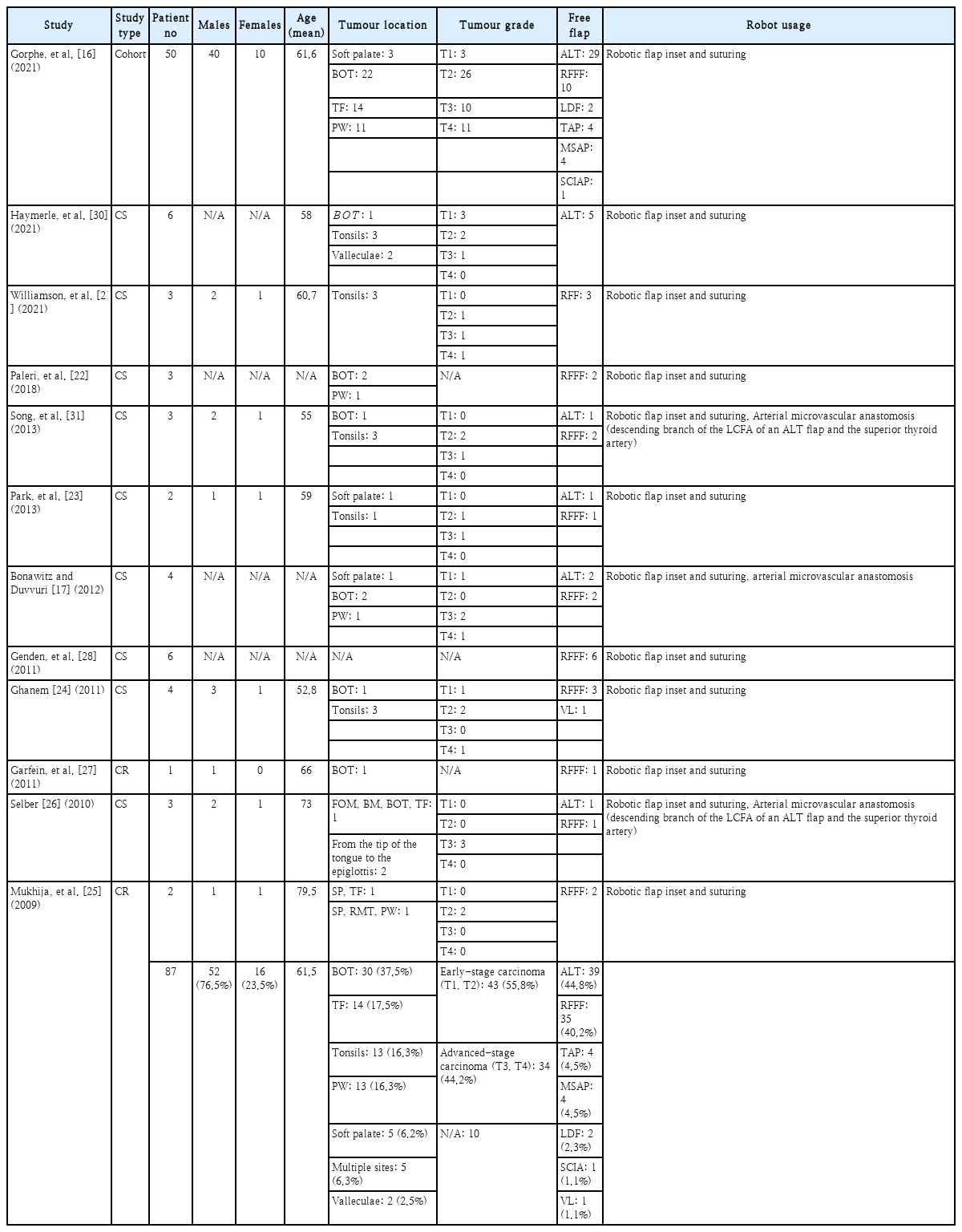
A total of 87 patients in 12 different studies were reviewed systematically. All studies were single-institution studies, either cohort studies, case series or case reports (level IV evidence). Robot-assisted reconstruction for early-stage oropharyngeal carcinoma (T1, T2) was done in a total of 43 patients, while 34 patients with advanced-stage oropharyngeal carcinoma (T3, T4) received oropharyngeal reconstruction. T-stage was not reported for the rest of the patients.
Flap selection
Following tumour removal, surgeons are often faced with the need to cover extensive and complex soft tissue deficits in the oropharyngeal region, aiming for preservation of the velopharyngeal sphincter, a watertight barrier between the oropharynx and the neck and provide sufficient sensation and bulk to the tongue base [12]. Free flaps are considered to have superior results and reduced complications over local or regional flaps, and they are preferred for this type of defects. Robot-assisted microsurgery, still being in its infancy especially regarding head and neck surgery, hasn’t been extensively studied and preliminary data are summarised in Table 1.
Current literature on robot-assisted free flap reconstruction of the oropharynx shows that two types of free flaps prevail: the radial forearm free flap (RFFF) and the anterolateral thigh (ALT) flap. More specifically, a total of 39 (44.8%) oropharyngeal reconstructions were carried out using an ALT flap, 35 (40.2%) patients received RFFF reconstruction (Table 1).
ALT flap
The ALT flap was first developed by Song, et al. [13]. It relies on perforators of the descending branch of the lateral circumflex femoral artery (LCFA) for arterial perfusion and venae commitante for venous outflow. Innervation is through the lateral femoral cutaneous nerve, useful for sensory restoration.
The flap’s thickness can be thinned safely to 4 mm for oropharyngeal reconstruction [14]. Variability in the flap’s vascular supply makes it more challenging to harvest, but the ALT flap’s long vascular pedicle, low donor site morbidity, and two-team approach make it a popular choice in head and neck reconstruction [15].
Fewer studies have selected the ALT flap for robot-assisted reconstruction [16] and, but Bonawitz and Duvvuri [17] recommend it for patients requiring bulk restoration after tumor resection at the base of the tongue.
RFFF
The RFFF was described initially in China by Yang in 1981 [18]. as thin and pliable flap. Its vascular network consists of the radial artery, with venous outflow either from the deep or superficial venous network through the cephalic vein. Its innervation comes from the forearm’s lateral cutaneous nerve.
The RFFF is considered a reliable free flaps, easily harvested, with a long vascular pedicle appropriate for anastomosis with neck vessels [19]. The main drawback is its association with increased donor site morbidity [20] as well as wound healing problems, tendon exposure, reduced mobility or sensation of the wrist and suboptimal cosmetic results. Nevertheless, the superficial harvest technique can alleviate donor-site complications, preserving the deep fascia of the forearm [14].
Concerning robot-assisted free flap reconstruction, all studies included in the review reported RFFF reconstructions, based on its structure which is well vascularised, thin, and pliable [17,21-24]. This could offer advantages in flap inset,coverage of the defect as well as superior wound healing, because of the flap’s good blood supply [21]. Compared to local flaps, Bonawitz and Duvvuri [17] specifically chose RFFF for palatal defects larger than 2 cm.
Robot-assisted flap inset
According to the literature, Mukhija, et al. [25] were the first to describe a robot-assisted reconstruction in two different cases; after TORS resection of a soft palate and tonsillar fossa lesion and TORS resection of recurrent carcinoma of the retromolar trigone extending to the palate and pharynx. In both cases, RFFF was used and the inset of the flap as well as suturing was done robotically, highlighting the potential of robotic reconstructive surgery in reducing overall operating time and avoiding mandibulotomy. The authors also reported good functional results in terms of speech and swallowing, although not going into detail about follow up time and methods for assessing these results.
Selber [26] reported 2 cases of using the da Vinci robot for insetting and suturing an RFFF and ALT flap for recurrent, advanced oropharyngeal carcinoma. The first patient’s oropharyngeal carcinoma affected the floor of the mouth, the buccal mucosa, the base of the tongue and the tonsillar fossa. Following tumour resection using TORS and pull-through technique, the flap was placed transorally to the defect area and sutured in place robotically using two 5-mm needle drivers. In a different case where the carcinoma extended from the tip of the tongue to the epiglottis, a two-paddle ALT flap was used to cover the defect area of the tongue, mouth floor, and pharynx and resurface the neck. Access anteriorly through the mouth and from the neck through a pharyngotomy, allowed for a hand-inset of the flap, whereas for the middle, robot assistance was used, again with two 5 mm needle drivers. It is apparent through the last case that hard to reach, and restricted areas of the neck could be benefited from the improved visualisation and precision of the robotic system. Park, et al. [23] who reconstructed 7 patients with oral and oropharyngeal cancer using ALT flaps after a TORS resection, also noticed that suturing deeper, difficult to reach parts of the neck; BOT, tonsil; required robotic assistance, whereas anterior reconstruction in the floor of the mouth could be easily performed using standard techniques.
Similarly, Ghanem [24] robotically sutured three RFFF and one vastus lateralis free flap using two 5-mm needle drivers in the oropharynx, reporting some more details on the robotic camera they used for reconstruction. More specifically, they found that a 30-degrees scope was helpful for the BOT and inferior pharyngeal regions while the 0-degree scope was suitable for the tonsils and soft palate. In the same year, Garfein, et al. [27] described a case where they circumferentially sutured an RFFF flap in the site of the BOT using the da Vinci robot and two 8-mm needle drivers. Although Garfein et al. reported no particular difficulty using the 8-mm needle drivers, Paleri, et al. [22] consider 8 mm needle drivers limiting in terms of arm movement in confined spaces such as the inferior pharynx.
Genden, et al. [28] published a case series of oropharyngeal reconstruction mainly using a local musculomucosal flap. Six patients, however, received an RFFF either because they have been previously treated with radiation therapy and they had an extensive oropharyngeal carcinoma involving the lateral pharynx, BOT and soft palate, or they only had extensive defects areas that could not be covered with local flaps. Previously irradiated fields are considered to be less vascularised and friable thus, making the free flap reconstruction necessary. All free flap insets and suturing were performed robotically, but micro-anastomosis was done with the standard technique. The authors explained, that for suturing the flaps, the wristed robotic arms provided them with increased movement freedom [29]. Additionally, the 30-degree robotic camera together with additional manual retraction helped suturing and visualisation in the inferior parts of the oropharynx.
Haymerle, et al. [30] recently published their experience with 5 ALT flaps and 1 RFFF flap for robotic reconstruction after removal of primary or recurrent carcinoma in the BOT, tonsils and valleculae. The authors shared some details on their reconstruction technique. Following the flap inset transorally and the inset of the flap pedicle to the neck for micro-anastomosing the vessels, stay sutures were placed first using the MegaTM Needle Driver. The rest of the sutures were placed as interrupted sutures or continuous sutures using V-Loc for the oropharyngeal sites, where it was difficult to tie knots. Microanastomosis of the vessels was also intentionally performed after the robotic inset to avoid swelling and bleeding which could affect visibility.
The largest and latest cohort study to be published yet on robot-assisted free flap reconstruction was conducted by Gorphe, et al. [16] that involved 50 patients. Free flaps being used ranged from ALT flaps to RFFF, latissimus dorsi flap, thoracodorsal artery perforator, medial sural artery perforator flap (MSAP) and superficial circumflex iliac artery perforator flap. During reconstruction, the flap inset was done first, so the flap was inserted transorally and the flap pedicle was guided to the neck with a pharyngotomy. The first stitches were placed in the upper part of the flap in case of tonsillar fossa and posterior pharyngeal wall reconstruction, and in the lower part in case of tongue base reconstruction. The da Vinci Robot was useful in placing sutures below the upper border of the suprahyoid epiglottis. All micro-anastomoses in this cohort of patients were completed by conventional methods.
Robotic microsurgical anastomosis
A very limited number of surgeons chose to perform microvascular anastomosis for oropharyngeal reconstruction using the robotic system. The continuous development of the robotic systems, their visualisation and magnification abilities, the precision and the improvement of instrumentation would certainly help the establishment of robotic microsurgical techniques.
The first robotic microsurgical anastomosis was reported by Selber [26], between the descending branch of the LCFA of an ALT flap and the superior thyroid artery, about 2 mm in diameter. The patient receiving robot-assisted reconstruction suffered from an advanced stage oropharyngeal carcinoma extending from the tip of the tongue to the epiglottis. For micro-anastomosis, black diamond needle holders were used, working synergistically to place the fine 9-0 sutures. The authors described that no leak was as well as no thrombosis was observed. Moreover, the back wall wasn’t caught between stitches and there was no need for hand-thrown stitches to be placed. Similarly, an arterial anastomosis of an ALT flap following TORS resection of a tongue base defect using the da Vinci surgical system was also reported by Bonawitz and Duvvuri [17]. Reconstructive surgeons used 2 black diamond needle holders to complete the anastomosis. The authors highlight, however, the need for the development of more microsurgery-oriented instruments such as a coupling system.
Song, et al. [31] were one of the three studies found in the literature to have reported robot-assisted microvascular anastomosis in one patient. The authors performed a TORS resection of a T3 stage tonsil carcinoma and a retro-auricular robot-assisted neck dissection. This approach resulted in the limited visibility of the facial artery which would be used for anastomosis with the radial artery of the RFFF, so they chose to use the robotic system to avoid extending the neck incision. Robotic instruments used for the microvascular anastomosis were black diamond forceps and Pots Scissors which have finer tips for placing micro-sutures. According to the authors, a robotic micro-anastomosis would be ideal for narrow places with hindered visualisation such as the retro-auricular incision which is primarily performed to avoid an extended scar of the traditional neck incision. One hundred fifty minutes were needed to perform the anastomosis, probably due to the steep learning curve involved in robotic microsurgery.
Complications
The success of surgical intervention largely depends on the safety of the procedure and the complications that arise. The free flap head and neck reconstruction has proved to be a safe procedure, with success rates ranging from 95% to 99%. There’s always a risk, however, of complications that could compromise the flap, exposing the underlying vessels and developing pharyngocutaneous fistulas [32]. Complications either by robot-assisted free flap reconstruction or by the traditional open-surgery free flap reconstruction are similar. What should interest us is if they occur at comparable rates, making the surgical system safe for performing these procedures. In the current literature, complications that have been reported include partial flap failure, flap dehiscence, postoperative haemorrhage, flap infection, fistula formation as well as a complete failure, delirium and pneumonia which caused the death of 2 patients.
The majority of studies included in the review reported no cases of significant robot-assisted reconstruction complications postoperatively at the time of follow up [17,22,23,25-28,31]. Gorphe, et al. [16], have reported results on the most extensive, to date, cohort of patients undergoing robotic oropharyngeal reconstruction, which have also encountered a greater number of complications. Authors also consider that the high number of previously irradiated patients at a rate of 60% has also contributed to the increased complication risk. Notably, they were the only ones to have reported complete flap failures. These four patients who received 2 ALT flaps, an RFFF flap MSAP flap had to be reoperated and their free flap had to be replaced. An additional three patients were reoperated for flap dehiscence and two for haemorrhage. Older studies have only encountered minor complications such as partial flap dehiscence, wound infections, and minor episodes of haemorrhage. Pharyngocutaneous fistula formation due to TORS tumour resection, paired with neck dissection is not uncommon but treatable [17,33]. In the case of robot-assisted free flap reconstruction, neck dissection is commonly used for passing the vascular pedicle of the flap and performing anastomosis with neck vessels, so this type of fistulas are to be expected. No study however has reported a pharyngocutaneous fistula. The only fistula formation that was reported was by Williamson, et al. [21]. The aforementioned fistula was a tracheocutaneous fistula following decannulation. Fig. 2 summarises the percentages of patients that presented these complications.
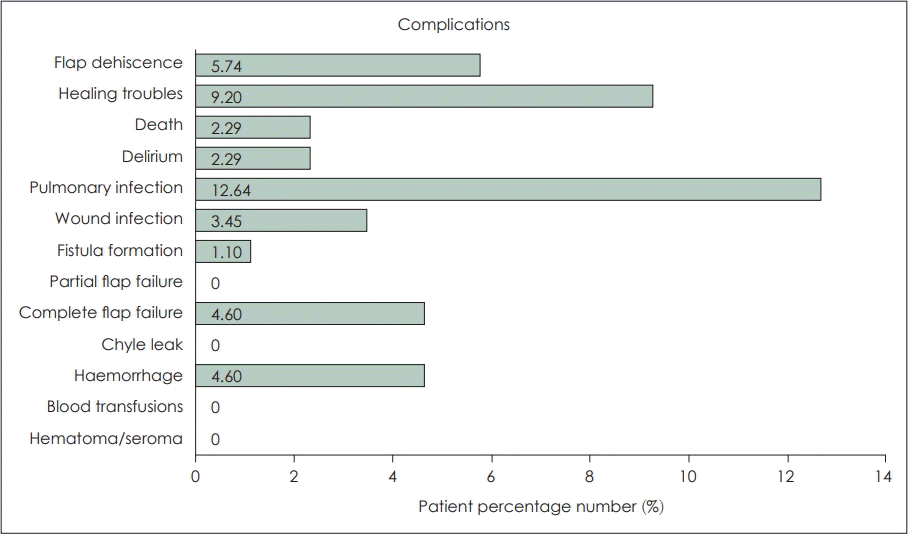
Summary of complications reported with robotic free flap reconstruction of oropharyngeal cancer defects.
Out of the 87 included patients, 42% suffered complications following tumour resection and robotic free flap reconstruction. Nevertheless, only 10% had to be reoperated and 4.6% had a complete flap failure. Of course, these numbers are completely indicative, as none of those studies was comparative, there were no randomised controlled trials, each study followed up patients for a different and short amount of time, and the number of patients being reported is comparatively small.
Treatment outcomes
Outcomes of robotic oropharyngeal reconstruction are not discussed or investigated in depth in the 12 included studies included. In terms of swallowing and speech performance postoperatively, results are heterogeneous and mainly subjective. Most studies include, however, some data on operative times, patient hospital stay postoperatively, days of resumption of oral diet postoperatively and patient follow up times (Table 2).
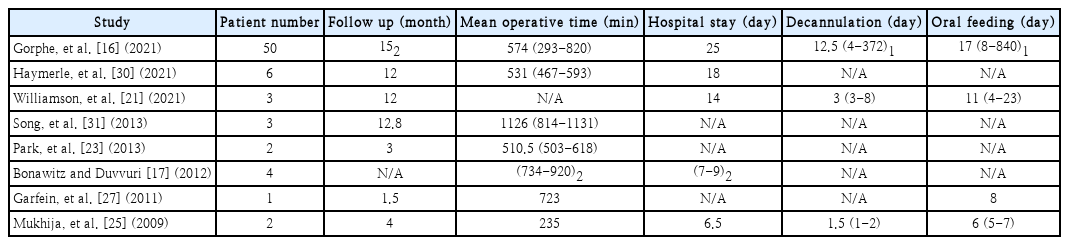
Summary table of studies reporting operative times, days of hospital stay, decannulation day, oral feeding resumption day
Haymerle, et al. [30] and Williamson, et al. [21] were the only ones who assessed the quality of life of patients undergoing robotic reconstruction regarding swallowing and performance. Haymerle, et al. [30] assessed patients using the MD Anderons Dysphagia Inventory (MDADI), Performance Status Score for Head And Neck and Penetration Aspiration Score whereas Williamson, et al. [21] used MDADI and University of Washington Quality of Life. Both studies reported no significant changes according to the patients’ answers to these questionnaires. The small number of patients assessed and the subjective nature of questionnaires, don’t let us draw definitive conclusions concerning swallowing and performance. Indicatively, objective evaluation tools that could be used to produce more robust results are the Iowa Oral performance instrument which measures the pressure generated by the tongue, and the modified barium swallowing procedure which assesses the oropharyngeal swallowing [34].
Operation time is another factor that should be assessed and in case robot-assisted oropharyngeal reconstruction is to be established. Some studies shared their mean total operation times which include robot docking, tumour resection, neck dissection and reconstruction (Table 2). The average operation time of these five studies in operations performed in 63 patients in total, is 9 hours and 42 minutes. Details about docking and reconstruction times were also shared by Park, et al. [23] in two patients with oropharyngeal cancer. Docking needed 13 minutes on average whereas reconstruction needed 315 minutes.
The day of decannulation, oral diet resumption and the hospital stay could also provide some insights into the effectiveness of the robotic approach. As most studies reported either a mean or a median number of days for these factors, Table 2 summarises their results. Gorphe, et al. [16] also measured the ratio of persistent enteral feeding after the last follow-up of patients at 30%. They attributed their results, among others, to the restoration of the volume of the base of the tongue, to the sensitivity preservation of the oropharynx and hypopharynx and the preservation of the lateral pharyngeal fold. Reconstruction of the posterior pharyngeal wall also shows the highest tendency for persisted enteral feeding.
Discussion
The robotic system is commonly used for free flap inset and suturing mainly because of the robot’s ability to reach transorally, anatomical sites deeper in the oropharynx. The augmented visualisation and illumination, the robot’s articulated arms which allow a precise and wider range of motion are the characteristics which according to many authors are important for oropharyngeal reconstruction. Following a TORS tumour resection, however, a free flap reconstruction could be done without robot assistance. Biron, et al. [35] in their case-control study state that they haven’t used the robot for any part of free flap reconstruction for patients with mainly early-stage oropharyngeal carcinoma. As surgeons get more exposure, experience and skills on the robotic systems and TORS is used for more complex cases of oropharyngeal cancer, the robotic system could prove to be an asset to oropharyngeal reconstruction.
Microsurgical anastomosis is a crucial part of a free-flap reconstruction in which the robotic system shows promise. A very limited number of surgeons in the current study selection have performed an arterial robot-assisted micro-anastomosis probably due to concerns of tissue manipulation without any haptic feedback, and the risk of vessel tear. Despite the important advantages of robotic systems such as tremor removal and movement precision, they still lack haptic feedback, and no technology so far has been able to circumvent this obstacle. All micro-anastomoses however were successful, without any vessel related issues such as anastomotic leaks. Every type of free flap is at risk of failure primarily because of thrombosis, making tissue handling during microsurgical anastomosis crucial [36]. During microsurgery, the gentle handling of the vessels, the preparation of the vessels by removing adventitia and the meticulous suturing define the viability of a flap so the robotic system should be able to perfectly perform these tasks. The development of more delicate instruments, vessel coupling systems, improved magnification capabilities which are currently inferior to the microsurgical microscope, the refinement of robotic microsurgical techniques, the detailed documentation of the limitations surgeons encounter would surely increase confidence in performing reconstructive procedures and could lead to the replacement of classic microsurgical approaches using the operating microscope.
In an effort to improve functional outcomes in patients undergoing oropharyngeal reconstruction, several studies examined the use of sensate free flaps and insensate free flaps [37]. No consensus exists however in the use of sensate flaps as well as the measurement of patient satisfaction, swallowing and speech performance. Whether or not sensate flaps prove to be important in improving functional outcomes, current literature on robot-assisted, oropharyngeal free flap reconstruction has not reported any sensory reinnervation of a free flap. With the increased use of robotic systems for microsurgical procedures and the advancement of robotic equipment and capabilities, the use of sensate free flaps could be explored and used to improve functional outcomes.
Cost evaluation of the robot-assisted free flap reconstruction was not mentioned in any of the studies included in the review, probably due to the novelty of the technique, and the limited amount of available data. Naturally, comparisons between traditional reconstruction techniques could not be made. Of course, multiple factors could affect the final cost of a robotic procedure. On the one hand, specialised training of surgeons and staff, the capital cost of the robotic system itself which reaches 2.5 million pounds and high yearly maintenance costs seem to increase the cost of a robot-assisted procedure [38]. Reduced hospital stay, complications and improved functional outcomes could, however, counterbalance the additional costs. More comparative studies which assess the above factors, and which conduct a detailed cost analysis of the procedure would certainly help to establish this new technique.
Of course, literature about this topic is scarce, and studies selected for reviewing show limitations. Available studies are in the form of case reports and small case series which have important disadvantages when we try to generalise data to a larger population. The heterogeneity of patient characteristics and outcomes, the short follow-up of patients only indicate that robot-assisted oropharyngeal reconstruction could be a safe procedure with minimal complications and improved functional results. Additionally, all studies do not compare robot-assisted reconstruction to a control group of patients who receive free flap reconstruction without the use of a robot. Thus, it is clear, that additional comparative studies, and randomised controlled trials with a more detailed description of the techniques being used and a more comprehensive analysis of the outcomes, would be needed to safely interpret data and conclude if the use of the robotic system offers superior results to open-approach procedures.
Conclusion
Free flap reconstruction of the oropharynx with robot assistance is an exciting new technique, that could expand the intraoperative abilities of a surgeon and offer improved functional and cosmetic results for the patients. Considering the novelty of this approach, few studies have explored robot assistance for oropharyngeal reconstruction. Comparative, multiinstitutional studies that involve a higher number of patients are still missing, and more information on long-term complications, functional results for the patients, details about reconstruction techniques are needed. These preliminary data, however, show the feasibility of robot usage for flap inset, suturing and micro-anastomosis of vessels, with no considerable short-term complications.
Acknowledgements
None
Notes
Author contributions
Conceptualization: Raghav C Dwivedi. Data curation: Panagiotis Vrakas. Formal analysis: Panagiotis Vrakas, Raghav C Dwivedi. Methodology: all authors. Project administration: all authors. Supervision: all authors. Visualization: Panagiotis Vrakas. Writing—original draft: Panagiotis Vrakas, Raghav C Dwivedi. Writing—review & editing: all authors.