 |
 |
AbstractImmunoglobulin G4-related disease (IgG4-RD) can involve multiple sites throughout the body. The most commonly affected sites in the head and neck are the salivary and lacrimal glands, respectively. Very few cases of IgG4-RD manifesting in the tonsils have been reported. Herein, we report the imaging findings of a 49-year-old female with pathologically confirmed IgG4-RD after a right-side tonsillectomy. The lesion showed diffuse homogeneous enhancement and appeared isointense and slightly hyperintense on T1- and T2-weighted magnetic resonance imaging, respectively. Diffusion restriction was also observed, which was considered to be due to the large number of IgG4-rich plasmacytes, and could vary depending on the degree of fibrosis. As distinguishing IgG4-RD from other diseases based solely on imaging is challenging, it is important to consider differential diagnosis, and clinical considerations are essential.
IntroductionImmunoglobulin G4-related disease (IgG4-RD) can involve multiple sites throughout the body and cause sclerosis and tissue inflammation by the infiltration of immune cells, generally resulting in a mass-like appearance; however, it can sometimes have an infiltrative appearance [1,2]. Therefore, it is necessary to differentiate these diseases from other malignancies. Precise identification of IgG4-RD is essential because, despite the potential for severe morbidity, the condition is treatable using glucocorticoids and rituximab [3]. However, IgG4-RD becomes resistant to treatment the longer it remains untreated, due to the amelioration from cellular infiltration to predominant fibrosis in tissues [4].
After the pancreas, the most common sites of involvement are the head and neck, usually in the maxillary sinus, lacrimal, and salivary glands. The tonsils are very rarely involved [5]. This case study aims to describe the imaging features of IgG4-RD, primarily involving the palatine tonsil, and to review the literature.
CaseA 49-year-old female presented with a 3-month history of foreign-body sensation in the throat and a progressively enlarged tonsillar mass on the right side. She did not complain of febrile sense, sore throat, cough, odynophagia, or dysphagia. She was healthy, and her medical history was unremarkable. She had no history of smoking or alcohol consumption. Endoscopic examination from the nose to the larynx revealed no pathological lesions, except for a large, protruding, lobulated tonsillar mass on the right side (Fig. 1A). The results of the blood laboratory tests, including C-reactive protein levels, were within normal ranges. CT (Fig. 2) and MRI (Fig. 3) revealed a protruding mass of approximately 25×29×16 mm confined to the tonsillar fossa on the right side, without evidence of involvement of the tongue, base of the tongue, or floor of the mouth. Enlarged, Level II lymph nodes suspicious for reactive hyperplasia were observed on both sides. No pathological lesions were observed in the salivary or the lacrimal glands. Ultrasonography-guided core needle biopsy was performed on the enlarged right lymph node. A biopsy of the tonsil mass on the right side was performed under local anesthesia. Benign lymphoid lesions without definite malignant findings were identified in the tonsil and lymph nodes. We performed a right tonsillectomy under general anesthesia. A McIvor Mouth Gag (Karl Storz, Tuttlingen, Germany) was used to expose the tonsils. A protruding tonsillar mass was observed on the right side of the confined tonsillar fossa. The mass and tonsil were resected via capsular dissection and bleeding was controlled using bipolar electrocauterization (Fig. 1B). Benign inflammation and fibrotic lesions were identified using frozen section analysis. The patient was treated with antibiotics (amoxicillin and clavulanate) and acetaminophen for 3 days and remained in the hospital for 2 days post-surgery.
Histopathological examination and immunohistochemical (IHC) studies (Fig. 4) revealed dense fibrosis with lymphoplasmacytic infiltration, with 240 IgG4-positive cells per high-power field and a ratio of IgG4-positive cells to IgG-positive cells of 21%. Diffuse fibrosis with phlebitis and partial obliterative changes were observed. When the revised comprehensive diagnostic criteria (RCD) proposed in 2020 were applied, these findings were considered compatible with the “probable IgG4-RD of the right tonsil” classification [2]. She refused further systemic evaluation for whole-body involvement and treatment with glucocorticoids because the tonsil mass and foreign body sensation in the throat were resolved by surgical treatment and there were no further complaints. At two-year follow-up, there was no symptom recurrence.
DiscussionIgG4-RD manifests as several historically recognized and characterized eponymous disorders that affect various organ systems. For instance, illnesses of the head and neck include unusual conditions now recognized as IgG4-RD symptoms, such as Riedel’s thyroiditis, Kuttner’s pseudotumor, and Mikulicz’s disease [3].
IgG4-RD is delineated by extensive infiltration of IgG4-positive plasma cells, storiform fibrosis, obliterative phlebitis, mild-to-moderate eosinophilia, and elevated count of IgG4-positive cells upon IHC staining [1,2]. According to the 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria (ACR/EULAR) for IgG4-related disease, the tonsil was not included in the diagnostic criteria; therefore, this patient did not meet the criteria for IgG4-RD [1]. Because the ACR/EULAR criteria excluded organs that were not frequently affected to maximize specificity, the RCD criteria for IgG4-RD were proposed in 2020 [2]. Thus, this patient was considered compatible with “probable IgG4-RD of the right tonsil,” according to the RCD criteria. Serum IgG4 concentration still has valuable diagnostic utility; however, our patient did not undergo this test, which limited the ability to apply the diagnostic criteria. However, approximately 21% of IgG4-RD patients do not exhibit elevated serum IgG4 levels [6]. Conversely, elevated serum IgG4 levels have been observed in other immune-mediated diseases [7].
While the presenting symptoms can vary based on the dominant organ involvement, constitutional symptoms are generally not observed [8]. Multiorgan involvement may occur in a synchronous or metachronous manner, and head and neck involvement is not necessarily associated with systemic disease [3]. Symptoms of IgG4-RD in the tonsils include throat pain and swelling, which do not respond to antibiotics or analgesics [9]. IgG4-RD is generally responsive to glucocorticoids, particularly in the early stages. However, up to 40% of patients may experience a subsequent relapse. Rituximab, a monoclonal anti-CD20 antibody, has demonstrated considerable efficacy, especially in instances of relapse or when diseases show resistance to glucocorticoid therapy [4]. Because the patient’s symptoms resolved after surgery, she refused further evaluation or glucocorticoid treatment. Clinically, no evidence of disease has been maintained for more than two-year post-surgery.
Only a few cases of IgG4-RD manifesting in the tonsils have been reported and, among these, studies reporting imaging findings are even rarer. Two case studies of IgG4-RD involving palatine tonsils were documented, however, imaging findings were not delineated [5,10]. In 2015, one case was reported affecting the tonsillar pillar, with the epicenter located at the left pharyngeal wall. The CT image showed soft tissue thickening with enhancement [11]. In 2016, a 59-year-old female with bilateral tonsillar enlargement was published focusing on radiologic findings including CT and MRI [9]. The lesions showed diffuse homogeneous enhancement and appeared isointense and slightly hyperintense on T1- and T2-weighted MRI, respectively, compared with the muscle. While diffusion-weighted imaging and apparent diffusion coefficient findings were absent among the MR sequences, the observations in our case were otherwise similar, except for the striated pattern seen in both tonsils on T2-weighted MRI. MR diffusion images involving the tonsils have not been reported, but a report on IgG4-RD involving the urethra observed diffusion restriction similar to that observed in our patient. This was attributed to a large number of IgG4-rich plasmacytes [12]. However, these results indicate that it is difficult to distinguish these lesions from malignancies, and that the results of diffusion restriction are expected to vary depending on the degree of fibrosis of the lesion. In 2020, a case of a 37-year-old female patient with a peritonsillar abscess was reported. The patient, who was initially treated for a peritonsillar abscess, exhibited a persistent mass, and histological examination confirmed the IgG4-RD diagnosis. The mass was successfully resolved with oral methylprednisone therapy. However, the report included no radiological findings [13].
Lesions associated with IgG4-RD are avid for 2-[18F]-fluoro-2-deoxy-D-glucose on positron-emission tomography (PET), a characteristic that can assist in the identification of multifocal asymptomatic disease, guide the planning of tissue biopsy, and monitor responses to treatment [14]. Differentiation from malignancy seems to eventually require a pathological examination. Unfortunately, our patient could not be evaluated with PET/CT.
In conclusion, clinicians should be attentive to the systemic manifestations of IgG4-RD and relevant differential diagnoses, as IgG4-RD occasionally mimics malignancy. Given the challenge of distinguishing IgG4-RD from other diseases based solely on imaging, histopathological confirmation and clinical considerations such as serum IgG4 levels and response to medication are essential.
ACKNOWLEDGMENTSThe study was conducted in compliance with the Institutional Review Board (IRB) regulations (approval ID: 2022-04-006) and the Declaration of Helsinki. The IRB approved a request to waive the need for informed consent.
Fig. 1.Representative preoperative endoscopic image (A) and tonsillectomy specimen (B). A: Laryngoscopy showing a large, protruding, and lobulated mass (asterisk) in the tonsil on the right side. B: The protruding tonsil mass arising on the tonsil is confined to the tonsillar fossa. The protruding tonsil mass and tonsil were resected via capsular dissection. Benign lymphoid lesions without definite malignant findings were identified in right tonsil mass through the biopsy under local anesthesia (arrow). R, right. 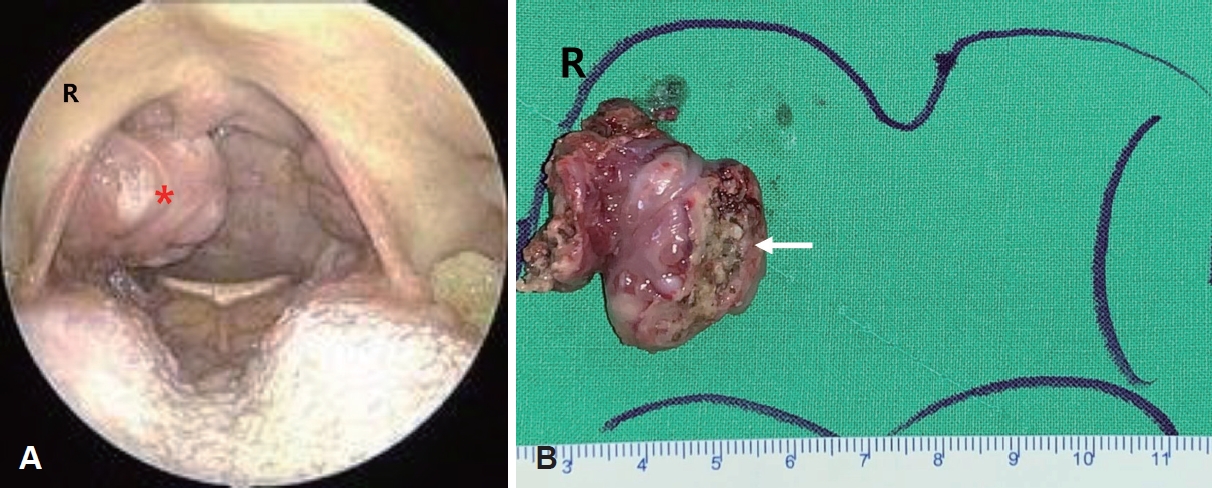
Fig. 2.Imaging finding of head and neck CT. Axial and coronal images showing an approximately 2.5 cm protruding isointense soft tissue lesion in the right tonsil (arrows). 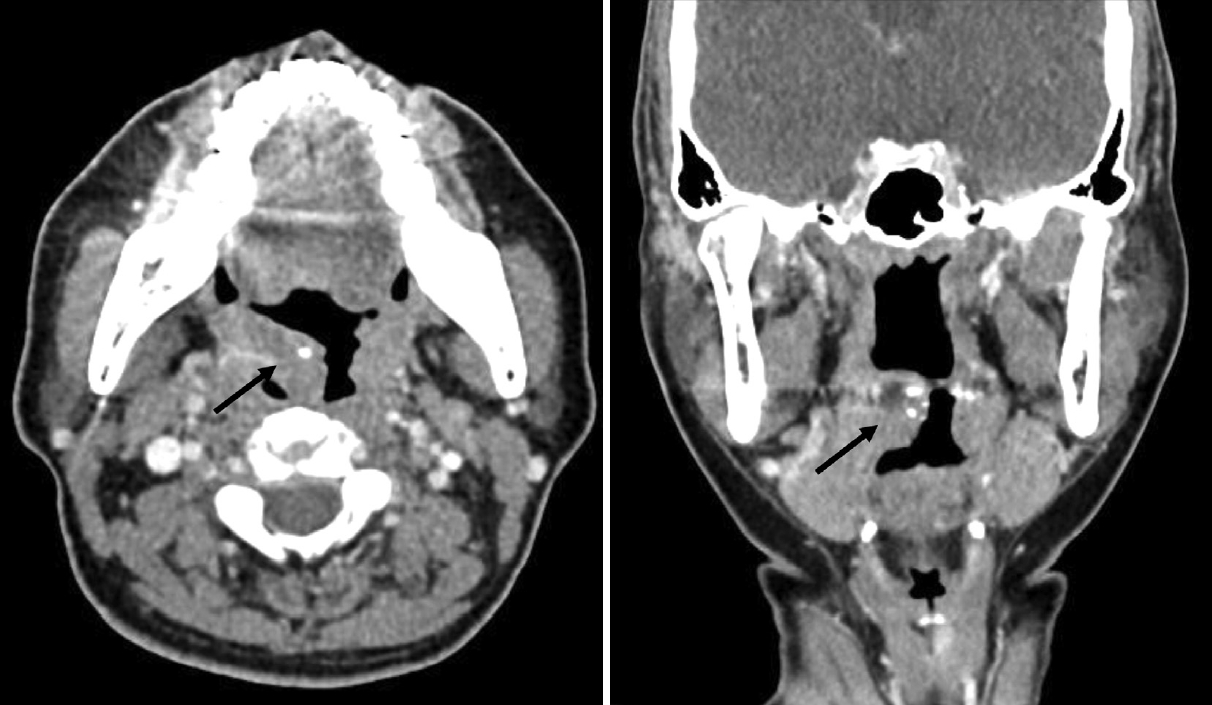
Fig. 3.Imaging finding of head and neck MRI. A and B: Coronal T1-weighted fast spin echo (A) and T2-weighted PROPELLER; (B) MR images demonstrating asymmetric bulging enlargement of the right palatine tonsil (arrows). Lesions appear isointense and slightly hyperintense on T1- and T2-weighted-images, respectively, compared to the muscle. C: Coronal Gadolinium enhanced T2-weighted image reveals diffuse enhancement in right tonsil (arrow). D and E: Diffuse weighted image b-800 shows a high signal, and the apparent diffusion coefficient shows a low signal in the right palatine tonsil (arrows). 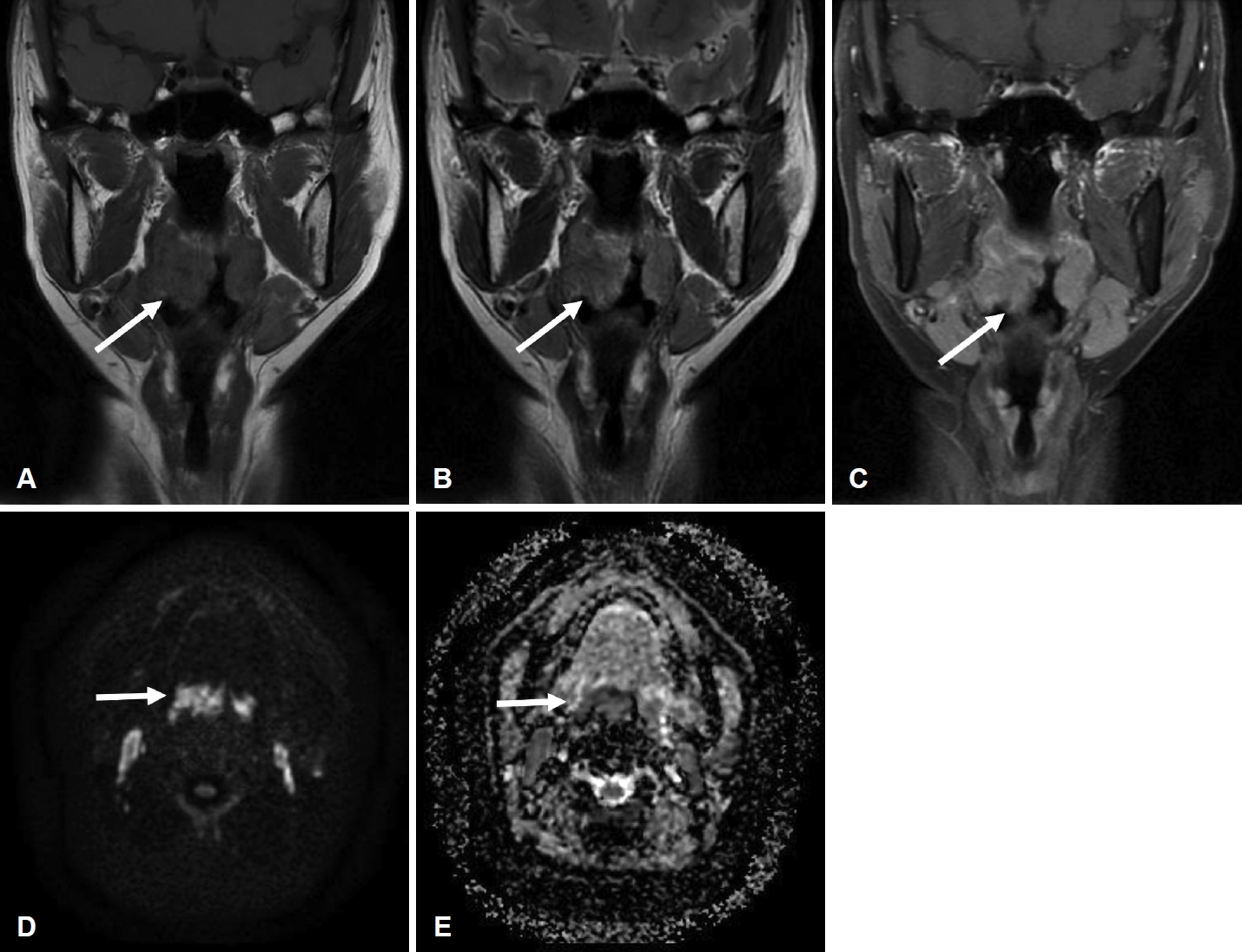
Fig. 4.Representative images of histopathological and immunohistochemical (IHC) studies of the tonsil mass. A and B: Microscopically, there was dense lymphoplasmacytic infiltration with diffuse marked fibrosis with a storiform pattern and phlebitis with partial obliterative change (A, hematoxylin and eosin staining [H&E] ×100 magnification; B, H&E ×200 magnification). C and D: On the IHC study images, immunoglobulin G (IgG)-positive cells (C) and IgG4-positive cells (D) were observed, and rich IgG4-positive cells per high-power field and a ratio of IgG4-positive cells to IgG-positive cells of 21% were identified (×200 magnification). 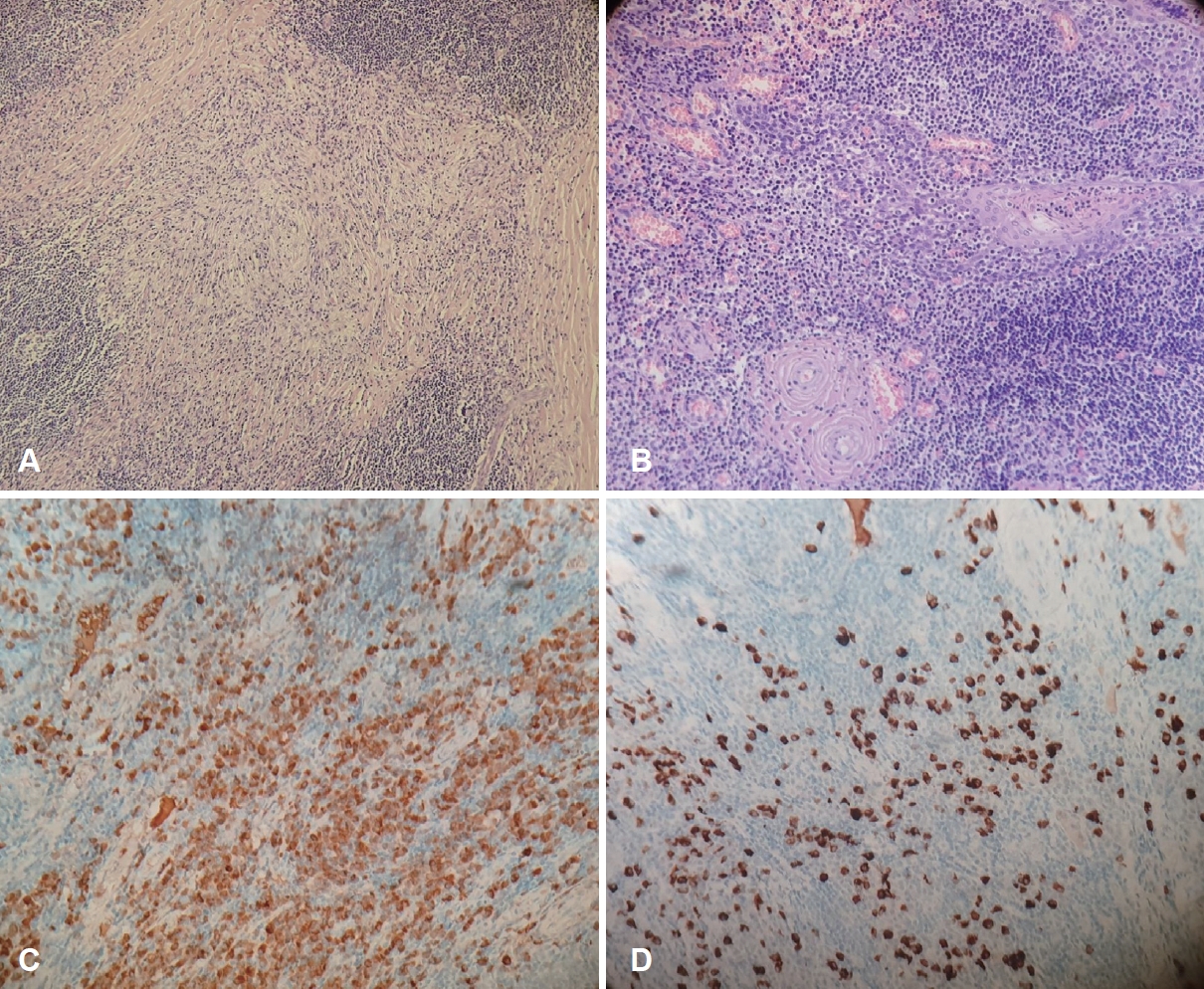
REFERENCES1. Wallace ZS, Naden RP, Chari S, Choi H, Della-Torre E, Dicaire JF, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Arthritis Rheumatol 2020;72(1):7-19.
2. Umehara H, Okazaki K, Kawa S, Takahashi H, Goto H, Matsui S, et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol 2021;31(3):529-33.
3. Thompson A, Whyte A. Imaging of IgG4-related disease of the head and neck. Clin Radiol 2018;73(1):106-20.
5. Khurram SA, Fernando M, Smith AT, Hunter KD. IgG4-related sclerosing disease clinically mimicking oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115(2):e48-51.
6. Tirelli G, Gardenal N, Gatto A, Quatela E, Del Piero GC. Head and neck immunoglobulin G4 related disease: systematic review. J Laryngol Otol 2018;132(12):1046-50.
7. Yamamoto M, Tabeya T, Naishiro Y, Yajima H, Ishigami K, Shimizu Y, et al. Value of serum IgG4 in the diagnosis of IgG4-related disease and in differentiation from rheumatic diseases and other diseases. Mod Rheumatol 2012;22(3):419-25.
8. Stone JH. IgG4-related disease: nomenclature, clinical features, and treatment. Semin Diagn Pathol 2012;29(4):177-90.
9. Park MH, Woo J Y, Lee Y, Yoon DY, Hong HS, Hong ME. Immunoglobulin G4-related sclerosing disease manifesting as bilateral tonsillar hypertrophy on MR images: a case report. Korean J Radiol 2016;17(1):147-50.
10. Chow TL, Yuen NW, Kwan WW, Choi CY. Immunoglobulin G4-related disease masquerading as tonsil carcinoma. Hong Kong Med J 2017;23(5):537-8.
11. Reder L, Della-Torre E, Stone JH, Mori M, Song P. Clinical manifestations of IgG4-related disease in the pharynx: case series and review of the literature. Ann Otol Rhinol Laryngol 2015;124(3):173-8.
12. Choi JW, Kim SY, Moon KC, Cho JY, Kim SH. Immunoglobulin G4-related sclerosing disease involving the urethra: case report. Korean J Radiol 2012;13(6):803-7.
|
|
||||||||||||||||||||||||||||||||||||||||||||

 |
 |