 |
 |
AbstractCochlear implants (CIs) are recognized as a safe and effective treatment for auditory rehabilitation for people of all ages with severe to profound sensorineural hearing loss. As the indications for CIs have expanded, recent advances in technology have focused mostly on preserving residual hearing. Because trauma to the internal structure of the cochlea can affect residual hearing, development has been focused on minimally invasive surgical procedures using robot technology along with the growth of interest in the field of CI surgery over the recent years. Maintaining a slow, steady rate of electrode insertion is widely accepted as an important factor associated with reduced intraoperative inner ear trauma and improved postoperative hearing outcomes. The use of robots have resulted in maintaining a slow rate of electrode insertion and reduced the degree of innate hand tremor in the movement of electrode array. We hereby present our experience of a successful robotic CI using iotaSOFT (iotaMotion, Inc.) and also present a review of the literature.
IntroductionCochlear implants (CIs) are electronic devices that directly stimulate spiral ganglion cells and the auditory nerve through an electrode array inserted into the cochlea. Cochlear implantation has been recognized as a safe and effective treatment for severe to profound bilateral or unilateral sensorineural hearing loss in people of all ages. Continued advances in CI surgical approaches and electrode designs have made it possible to preserve residual hearing. Preservation of hearing in the low-frequency region allows CI users to improve their listening abilities in complex listening environments, such as music, and speech understanding in noisy environments [1,2]. As the indications for CIs have expanded, recent advances in technology have largely focused on preserving residual hearing. It is recognized that damage to the internal structure of the cochlea due to mechanical forces such as electrode array insertion and drilling near the cochlea can affect residual hearing [3].
Robots have been developed since the 1980s for application in various aspects of surgery, with early successful adoption in urology, gynecology, and neurosurgery [4]. The successful use and feasibility of robots in otolaryngology has been demonstrated primarily in head and neck surgery [5]. Due to the confined microscopic anatomy of the middle ear and temporal bone and the delicate nature of surgery, there has been a movement to adopt robots in otology over the past decade, with CI surgery being the most frequently attempted robot-assisted surgery [6]. The goals of robotic CI surgery are to avoid overdrilling of the mastoid, to preserve residual hearing through more consistent insertion techniques, and to accommodate patients with complex anatomy such as middle and inner ear deformities [7].
Several robots have been developed for cochlear implantation. HEARO® (CAScination AG, Bern, Switzerland) is a robot that provides minimally invasive access from the temporal bone to the inner ear with an automated drill [8]. Around the same time, RobOtol® (Collin Medical, Bagneux, France) was also designed to reduce physical trauma during electrode insertion [9]. Slow and consistent electrode insertion rates are widely accepted as an important factor associated with reduced intraoperative inner ear trauma and may improve postoperative hearing outcomes. Electrode insertion can vary greatly between operators and depending on the patient’s anatomy and the type of electrode. Robotic cochlear implantation is beginning to gain traction as a tool to standardize the speed and trajectory of electrode insertion during surgery [10]. The iotaSOFT (iotaMotion, Inc., Iowa City, IA, USA) system, like the RobOtol® system, is a robotic device to assist with electrode array insertion that is FDA approved and available in the US market. The authors have successfully performed cochlear implantation using the iotaSOFT robot and would like to present our experience and surgical approach along with a review of the literature.
MethodsRobot-assisted electrode insertion device1) Control console (Fig. 1A and B)
2) Foot pedal (Fig. 1C)
3) Drive unit (Fig. 1D)
Mastoidetomy and posterior tympanotomyUnder general anesthesia, a skin incision is made that should extend superior to the auricle to allow for enough pars squamosa of the temporal bone exposure for securing the drive base with two screws. A complete canal wall up mastoidectomy is performed with a wide posterior tympanotomy. The bony overhang covering the round window is removed to expose the round window membrane. A tight subperiosteal pocket is created to place the receiver/stimulator.
Installing and positioning the robotThe product consists of the control console, which is the user interface, the drive unit and base, which is secured to the skull and holds the electrode array, and the foot pedal, which controls the speed and direction of insertion. The control console is connected to the drive unit and foot pedal via USB ports. The drive arm of the drive unit, which serves as a guide for electrode insertion, is flexible enough to bend, and the drive head is rotatable, allowing the operator to customize the insertion position and ideal axis (Fig. 2A). The drive base, which acts as an anchor on the superior aspect of the mastoidectomy site, is placed in the appropriate position (Fig. 2B) and secured with two screws.
Robot-assisted electrode array insertionWhile adjusting the position of the drive unit, find the direction and position where surgeon wants to insert the electrode and open the drive head (Fig. 3A). The electrode array is placed in the guide track, and then the drive head is closed (Fig. 3B) to secure the electrode array prior to placing it on the drive base. The position of the drive head is fine adjusted to orient it towards the round window or cochleostomy (Fig. 3C). The pedal is then engaged to insert the electrode array while using a suction or claw instrument to guide it into the cochlea. The robot insertion speed and direction can be adjusted on the control console or on the pedal. The electrode array is inserted with an insertion speed between 0.1-0.4 mm/s (Fig. 3D). Once insertion is complete, the electrode array is immobilized with forceps and the drive head is opened to release the remaining electrode array (Fig. 3E). The drive head is then unlocked and removed followed by releasing the drive base from the skull by removing the screws that secured it to the skull (Fig. 3F).
This study has been granted an exemption by the Institutional Review Board (Y1-24-0147).
See Supplementary Video 1 demonstrating the procedure.
Results and DiscussionThe ultimate goal of CI surgery is to safely insert the electrode array into the cochlea as atraumatically as possible. Many aspects of electrode array design have been studied to optimize the auditory gain from CI surgery. These include the depth of insertion, proximity to the modiolus, and the insertion technique, including the force and speed with which the electrode array is inserted [11].
Numerous studies have reported better hearing and speech perception with atraumatic surgical techniques during CI surgery [12]. Avoiding damage to the inner ear, such as damage to the basilar membrane or lateral wall, electrode translocation from the scala tympani to the scala vestibuli, or fracture of the osseous spiral lamina, can reduce the risk of cochlear inflammation and oxidative stress, which can lead to the formation and progression of intracochlear fibrosis [13,14]. The entry point diameter of the scala tympani (1233 μm) is approximately twice the diameter of CI electrodes (500-800 μm), and insertion of the electrodes requires a smooth and precise motion to preserve low-frequency residual hearing [12]. However, the insertion process can be affected by involuntary movements such as hand tremor, even in experienced surgeons. Physiologic hand tremor is a natural occurrence in humans and can be exacerbated by muscle fatigue and emotional stress. In otolaryngologic surgery, it can be particularly pronounced, as the surgeon often uses slow hand movements while looking at small surgical areas under a microscope. Natural hand tremor can contribute to intracochlear trauma during CI surgery.
Over the past decade, there has been increasing interest in the use of robotics in CI surgery, with various centers investigating robotics to facilitate CI surgery. These include image guided surgical planning, guided keyhole drilling into the mastoid to access the middle or inner ear without extensive mastoidectomy, and electrode insertion robotic systems [11]. While iotaSOFT is the only FDA approved robotic system for cochlear implantation in the US, it is one of several robotic systems that have been utilized for this procedure. The following are notable advantages and disadvantages when iotaSOFT robot is compared to other robotic systems used in CI surgery. The iotaSOFT insertion system is a thumb-sized sterile device that operates with a single use, while other robotic CI system equipment requires relatively large equipment and space. In current practice, there are two major roboticsassistance platforms (RobOtol® system and iotaSOFT system) used for electrode array insertion. A study with robotics assistance using the RobOtol® system reported a mean preparation time of 630 s, however preparation of the iotaSOFT system required a mean duration of 55.8 s. Therefore the preparation time for surgery is relatively short [15,16]. Electrode migration can occur during the closing phase of surgery, postoperatively or later on. A possible stabilizing solution is the electrode lead fixation clip, a gentle groove between the facial nerve and chorda tympani, and fixing the electrode with bone dust mixed with fibrin glue [17,18]. iotaSOFT which insert the electrode through the classic CI approach with an open mastoid and posterior tympanotomy have the diverse same options as that of manual electrode insertion in stabilizing the electrode regardless of the electrode types. But robotic systems, such as HEARO®, Rosa® (Zimmer Biomet, Warsay, IN, USA), which drill a narrow tunnel from the cortex to the cochlea, need an alternate solution for stabilizing the electrode [19]. The RobOtol® system can be used for both straight electrode and perimodiolar electrode array insertion. However, the current iotaSOFT system is compatible with the straight electrode. Its next iteration is expected be compatible with both straight and perimodiolar electrodes [20].
Robotic insertion of electrodes has the potential to minimize intracochlear pressure fluctuations by reducing the insertion speed and simultaneously reducing the variations in speed of insertion [21]. Insertion speed is related to insertion forces, and the basilar membrane is easily damaged by forces as low as 0.029 newton [22]. Slow, constant insertion speeds have been shown to reduce intracochlear fluid forces, which may be important for preserving residual hearing by reducing intracochlear volume coverage and causing less mechanical damage to the basilar membrane and the orgran of Corti [11]. In temporal bone and animal model studies comparing robotic versus manual insertion, robotic insertion was associated with more precise insertion into the scala tympani and less inner ear trauma due to fewer electrodes being placed in the scala vestibuli [23]. In addition, slower insertion speeds with robotic insertion are associated with lower intracochlear forces, which is favorable for residual hearing preservation.
Although there is a range depending on the operator and the type of electrode, the lowest speed at which a human operator can reliably insert an electrode is known to be around 0.86 mm/s, with a real-world average insertion speed of 1.6 mm/s [22]. When considering overall intracochlear force, studies have generally found that the speed of electrode array insertion is directly and positively correlated with generation of intracochlear force. In a synthetic cochlea model, progressive increases in insertion speed resulted in greater average and maximum insertion force, with the lowest insertion force correlating with the lowest speed tested of 0.16 mm/s [22]. For comparison, the average speed of electrode array insertion for CI surgeons is 10 times higher at approximately 1.6 mm/s [22]. A study by Kesler, et al. [24] described that the lower limit of a constant forward motion manual electrode array insertion lies at an average speed of 0.87 mm/s and noted that a 0.25 mm/s insertion rate is not feasible for human operators to achieve. There is some evidence to suggest that slower insertion may improve postoperative clinical measures. Rajan, et al. [25] performed comparisons between patients implanted with a target ‘slow’ insertion speed of 0.25 mm/s vs. a ‘fast’ speed of 1 mm/s with the same electrode array. They found that patients undergoing slow insertion had significantly higher rates of postoperative hearing preservation, more complete electrode array insertions, and a decreased incidence of vestibular symptoms in a 24-hours period after implantation. Therefore, we are proceeding with electrode array insertion at a speed of 0.2 mm/s or less.
The overall operating time is clearly increased. Robotic CI surgery is more time-consuming compared to the average 80 to 90 minutes required by an experienced CI surgeon [21]. In the author’s case, the first robotic CI surgery resulted in a 40% increase in operative time. After the first surgery, the time decreased considerably, but there is a learning curve and subsequent decrease in operative time due to need for precise manipulation of the robotic equipment to screw in the electrodes, mount the electrodes to the driver unit, and position the electrode insertion distance and angle. So far we have performed three CI surgeries with the iotaSOFT robotic system, and more are scheduled for the coming weeks. In two cases, residual hearing were preserved, but in one case, air conduction thresholds were declined by an average of 20 dB on pure tone audiometry at 250 Hz and 500 Hz. In the declined case, acoustic trauma from the drill, disruption of inner ear homeostasis, and infection are more likely causes than possible physical trauma during electrode insertion. However, it is important to note that this was a small cases of <5 patients and was acknowledged to be underpowered to detect significant residual hearing preservation. Thus, larger studies are needed to evaluate the effectiveness of robotic-assisted cochlear implantation on patient’s residual hearing outcomes.
The robot’s high precision of movement and ability to insert the electrodes without involuntary movement allows for a slower, steady insertion speed compared to humans, resulting in a less traumatic insertion. On the one hand, the lack of tactile feedback during insertion by the robot may lead to situations such as kinking, bending, and rollover. However, comparisons of robotics-assisted insertion with manual insertion have demonstrated that robotics-assisted insertion is associated with reduced intracochlear force generation and rates of trauma, including tip fold-over [26]. The slow, but adjustable insertion rate allowed with the iotaSOFT system allows the surgeon to immediately stop or withdraw the electrode array when bending or kinking is identified. The trajectory of the electrode can easily be altered to a more favorable angle prior to restarting the insertion. If a tip fold-over is identified the iotaSOFT angle of insertion can be adjusted along with the rate of insertion to re-insert the electrode ideally with real-time fluoroscopy or electrocochleography. While slower speed may reduce insertion forces, there still exists a need to elicit feedback from the cochlea to prevent injury. Integration of electrochocleography responses or force measurements with a robotics-platform opens the possibility of automatically halting insertion based on feedback variables [27]. Also combining robotics-assisted electrode array insertion with navigation software that can optimally orient the electrode array for insertion into the round window would be?a strong adjunct, as insertion trajectory is considered a significant variable in force generation [28,29]. Precise assessment of insertional depth based on tonotopic estimates is another area of development. These approaches, along with intraoperative X-ray, fluoroscopy are expected to further reduce the incidence of complications due to lack of tactile feedback during electrode array insertion.
The field of robotic CI surgery has much potential for advancement in the future, as it can provide minimally invasive, precise, and personalized care. Nevertheless, there are significant challenges that need to be overcome and demonstrated, such as safety, efficiency, surgical time, and cost, before this approach can become an acceptable alternative to traditional CI insertion techniques Prospective clinical trials will be needed to evaluate the reduction in surgery-related complication rates and improvement in audiologic and functional outcomes compared to conventional CI surgery. While the total number of robotic CI cases performed is still limited and more research needs to be conducted to comprehensively evaluate its utility, the development of robotic CI surgery is an exciting and promising prospect and is expected to be the next step in the evolution of CI surgery.
Supplementary MaterialsThe Data Supplement is available with this article at https://doi.org/10.3342/kjorl-hns.2024.00192.
NotesAuthor contributions Conceptualization: Joe Walter Kutz Jr, Brandon Isaacson, Hyun Sang Cho. Data curation: Hyun Sang Cho. Formal analysis: Hyun Sang Cho. Software: So Young Ko. Supervision: Joe Walter Kutz Jr, Brandon Isaacson. Validation: Joe Walter Kutz Jr, Brandon Isaacson. Visualization: So Young Ko. Writing—original draft: Hyun Sang Cho. Writing—review & editing: Joe Walter Kutz Jr, Brandon Isaacson. Fig. 1.Overview of iotaSOFT (iotaMotion, Inc.). A and B: Control console. C: Foot pedal. D: Drive unit. 
Fig. 2.Drive unit and drive base. A: The arm of the drive unit is bendable and the head is rotatable, allowing the operator to manipulate the insertion position and axis. B: The drive base is secured to the temporal squamosa with two screws superior to the mastoid cavity. 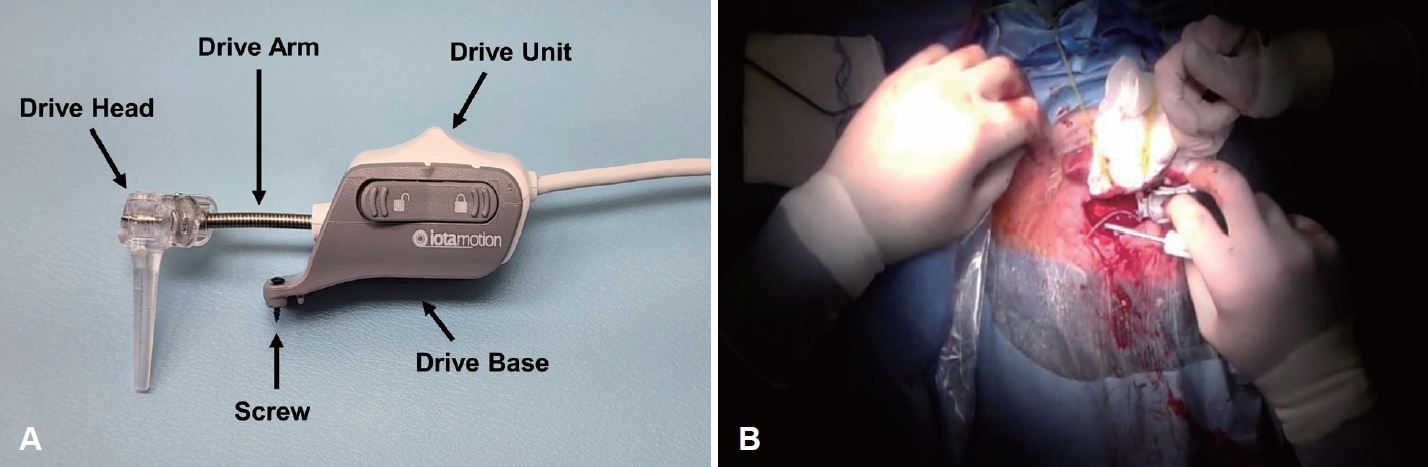
Fig. 3.Schematic photos of robot assisted cochlear implant electrode array insertion using iotaSOFT (iotaMotion, Inc.). A: The loading notch on the drive head is opened to place the electrode array into guide track. B: The drive unit is placed into the drive base and is locked into position. C: The device head is adjusted to orient the electrode array towards the cochleostomy. D: The foot pedal is engaged to insert the electrode array into the cochlea using an instrument to guide it towards the round window. E: Once the electrode array is fully inserted it is held in position with forceps. The drive head is opened to release the electrode array and is then removed from the drive base. F: The drive base is removed after removing the screws. 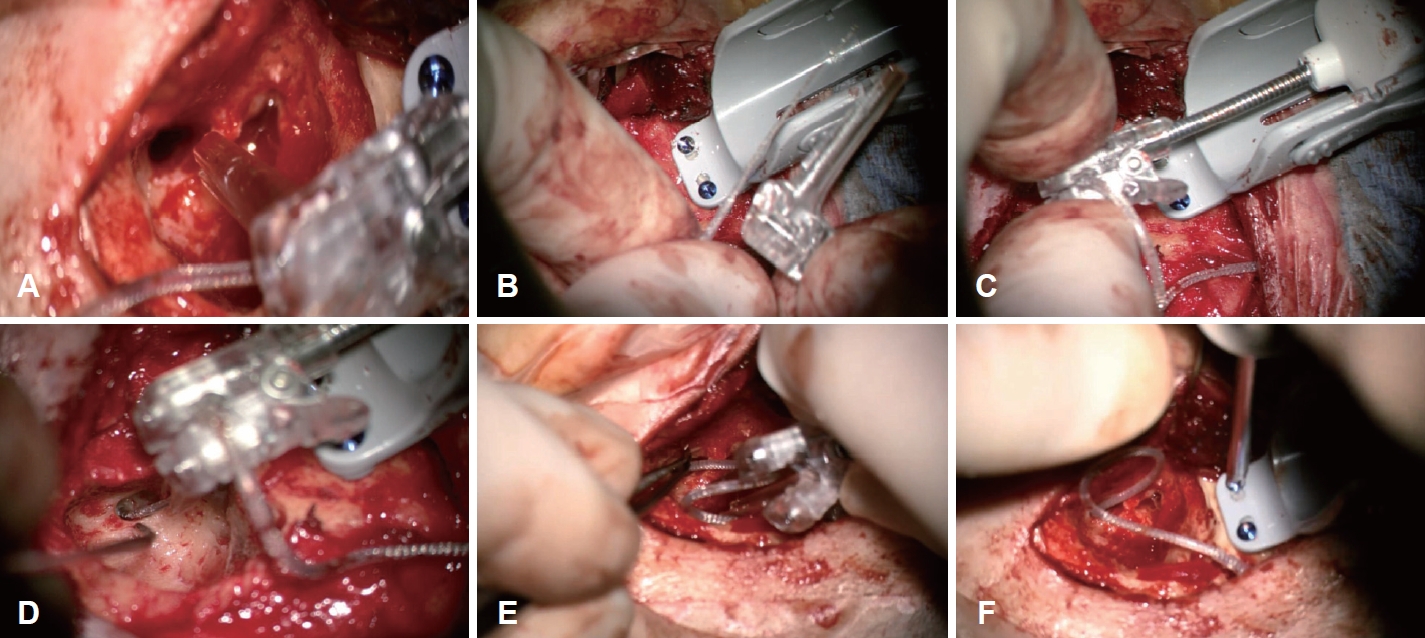
REFERENCES1. Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope 2005;115(5):796-802.
2. Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol 2006;11(Suppl 1):12-5.
3. Eshraghi AA, Ahmed J, Krysiak E, Ila K, Ashman P, Telischi FF, et al. Clinical, surgical, and electrical factors impacting residual hearing in cochlear implant surgery. Acta Otolaryngol 2017;137(4):384-8.
4. Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng 1988;35(2):153-60.
5. Tamaki A, Rocco JW, Ozer E. The future of robotic surgery in otolaryngology - head and neck surgery. Oral Oncol 2020;101:104510.
7. Auinger AB, Riss D, Baumgartner WD, Arnoldner C, Gstöttner W. Robot-assisted cochlear implant surgery in a patient with partial ossification of the basal cochlear turn: a technical note. Clin Otolaryngol 2022;47(3):504-7.
8. Topsakal V, Heuninck E, Matulic M, Tekin AM, Mertens G, Van Rompaey V, et al. First study in men evaluating a surgical robotic tool providing autonomous inner ear access for cochlear implantation. Front Neurol 2022;13:804507.
9. Barriat S, Peigneux N, Duran U, Camby S, Lefebvre PP. The use of a robot to insert an electrode array of cochlear implants in the cochlea: a feasibility study and preliminary results. Audiol Neurootol 2021;26(5):361-7.
10. Tarabichi O, Jensen M, Hansen MR. Advances in hearing preservation in cochlear implant surgery. Curr Opin Otolaryngol Head Neck Surg 2021;29(5):385-90.
11. Shoman NM. Robotics and cochlear implant surgery: goals and developments. Curr Opin Otolaryngol Head Neck Surg 2022;30(5):314-9.
12. Daoudi H, Lahlou G, Torres R, Sterkers O, Lefeuvre V, Ferrary E, et al. Robot-assisted cochlear implant electrode array insertion in adults: a comparative study with manual insertion. Otol Neurotol 2021;42(4):e438-44.
13. Eshraghi AA, Van de Water TR. Cochlear implantation trauma and noise-induced hearing loss: apoptosis and therapeutic strategies. Anat Rec A Discov Mol Cell Evol Biol 2006;288(4):473-81.
14. Bas E, Dinh CT, Garnham C, Polak M, Van de Water TR. Conservation of hearing and protection of hair cells in cochlear implant patients’ with residual hearing. Anat Rec (Hoboken) 2012;295(11):1909-27.
15. Maheo C, Marie A, Torres R, Archutick J, Leclère JC, Marianowski R. Robot-assisted and manual cochlear implantation: an intraindividual study of speech recognition. J Clin Med 2023;12(20):6580.
16. Gantz JA, Gantz BJ, Kaufmann CR, Henslee AM, Dunn CC, Hua X, et al. A steadier hand: the first human clinical trial of a singleuse robotic-assisted surgical device for cochlear implant electrode array insertion. Otol Neurotol 2023;44(1):34-9.
17. Müller J, Schön F, Helms J. [Reliable fixation of cochlear implant electrode mountings in children and adults--initial experiences with a new titanium clip]. Laryngorhinootologie 1998;77(4):238-40, German.
18. Loth AG, Adel Y, Weiß R, Helbig S, Stöver T, Leinung M. Evaluation of a bone groove geometry for fixation of a cochlear implant electrode. Eur Arch Otorhinolaryngol 2020;277(2):385-92.
19. Van de Heyning P, Roland P, Lassaletta L, Agrawal S, Atlas M, Baumgartner WD, et al. Suitable electrode choice for robotic-assisted cochlear implant surgery: a systematic literature review of manual electrode insertion adverse events. Front Surg 2022;9:823219.
20. Jia H, Pan J, Gu W, Tan H, Chen Y, Zhang Z, et al. Robot-assisted electrode array insertion becomes available in pediatric cochlear implant recipients: first report and an intra-individual study. Front Surg 2021;8:695728.
21. Panara K, Shahal D, Mittal R, Eshraghi AA. Robotics for cochlear implantation surgery: challenges and opportunities. Otol Neurotol 2021;42(7):e825-35.
22. Kontorinis G, Lenarz T, Stöver T, Paasche G. Impact of the insertion speed of cochlear implant electrodes on the insertion forces. Otol Neurotol 2011;32(4):565-70.
23. Torres R, Jia H, Drouillard M, Bensimon JL, Sterkers O, Ferrary E, et al. An optimized robot-based technique for cochlear implantation to reduce array insertion trauma. Otolaryngol Head Neck Surg 2018;159(5):900-7.
24. Kesler K, Dillon NP, Fichera L, Labadie RF. Human kinematics of cochlear implant surgery: an investigation of insertion micro-motions and speed limitations. Otolaryngol Head Neck Surg 2017;157(3):493-8.
25. Rajan GP, Kontorinis G, Kuthubutheen J. The effects of insertion speed on inner ear function during cochlear implantation: a comparison study. Audiol Neurootol 2013;18(1):17-22.
26. Majdani O, Schurzig D, Hussong A, Rau T, Wittkopf J, Lenarz T, et al. Force measurement of insertion of cochlear implant electrode arrays in vitro: comparison of surgeon to automated insertion tool. Acta Otolaryngol 2010;130(1):31-6.
27. Gawęcki W, Balcerowiak A, Podlawska P, Borowska P, Gibasiewicz R, Szyfter W, et al. Robot-assisted electrode insertion in cochlear implantation controlled by intraoperative electrocochleography—a pilot study. J Clin Med 2022;11(23):7045.
|
|
||||||||||||||||||||||||||||||||||||||||||

 |
 |