 |
 |
AbstractBackground and ObjectivesElectronic cigarette (e-cigarette) is a device that generate vapor by heating e-cigarettes liquid. E-cigarette damages the respiratory immune system and renders the respiratory tract vulnerable to inflammations. However, there are no studies on how the inflammatory reactions in respiratory epithelial cells caused by e-cigarette occur, and the effects of Korean red ginseng (KRG) and saponin on inflammation induced by e-cigarette are unknown. This study aimed to compare the inflammatory reactions caused by e-cigarette and lipopolysaccharide (LPS), and to investigate the effects of KRG and saponin on cytokine and mucin expression induced by e-cigarette in respiratory epithelial cells.
Subjects and MethodIn bronchoalveolar lavage fluid and lung tissue of mice, the effects of KRG and saponin on cytokines (interleukin [IL]-1ќ≤, IL-6, IL-8) and mucin 5AC/5B (MUC5AC/5B) expression induced by e-cigarette were investigated using real-time polymerase chain reaction, enzyme linked immunosorbent assay, and immunohistochemistry staining.
ResultsInflammatory cells, cytokines (IL-1ќ≤, IL-6, IL-8) and MUC5AC/5B messenger RNA expression and protein production were increased by e-cigarette, similar to LPS. KRG and saponin decreased the expression of cytokines (IL-1ќ≤, IL-6, IL-8) and MUC5AC/5B induced by e-cigarette. KRG and saponin showed effects similar to that of dexamethasone.
ConclusionE-cigarette causes inflammation similar to that caused by LPS. KRG and saponin regulate the expression of cytokine and MUC5AC/5B increased by e-cigarette in respiratory epithelial cells. KRG and saponin may be an effective therapeutic option for inflammatory responses induced by e-cigarette in respiratory epithelial cells.
IntroductionElectronic cigarette (e-cigarette) have been widely used since the 2000s as a method of inhaling vapor made by heating the e-cigarette liquid unlike conventional cigarette. Initially e-cigarette is considered less harmful than conventional cigarette due to fewer harmful substances such as ash and tar, and has been used as an alternative to quit smoking. But, the harmful effects of e-cigarette in health and respiratory system have been continuously revealed [1,2]. E-cigarette is known to damage respiratory immunity and defense mechanisms, making respiratory tract vulnerable to inflammatory reactions [3-5].
Airway mucus protecting a respiratory tract is determined by the quality and quantity of mucin. Mucin gene expression is regulated by cytokines, reactive oxygen species, and other inflammatory mediators [6]. The expression of various mucin genes and cytokines is observed in inflammatory respiratory diseases [6,7]. Mucin 5AC/5B (MUC5AC/5B), as the representative secretory mucin genes and pro-inflammatory cytokines have been actively used in studies dealing with airway epithelium [6,8-10].
Korean red ginseng (KRG) (Panax ginseng Meyer) is a herbal medicine used in Korean traditional medicine. Panax is a combination of вАШpan-,вАЩ which means everything, and вАШaxos,вАЩ which means medicine. As it means a medicine that treats everything, KRG has been found to be effective in anti-inflammatory, anti-cancer, and anti-aging and used in various studies [11-14]. Among several substances that consist of KRG, such as saponin, polysaccharides, polyalkenes, polyacetylenes, vitamins, and fatty acids, saponin plays the most important role in anti-inflammatory reaction [15,16]. Recently, some studies have been conducted using KRG and saponin to control respiratory diseases [9,10,17-21].
Studies on how inflammatory reactions caused by e-cigarette occur, and the effects of KRG and saponin on inflammation induced by e-cigarette in respiratory epithelium were not studied. Anti-inflammatory reactions of KRG and saponin in respiratory epithelial cells have not been investigated enough compared to other organs.
Therefore, we compared inflammatory reactions caused by e-cigarette with lipopolysaccharide (LPS) through changes in cytokines and MUC5AC/5B in bronchoalveolar lavage (BAL) fluid and lung tissue of experimental mice. Also, we evaluated the effects of KRG and saponin on pro-inflammatory cytokines (interleukin [IL]-1ќ≤, IL-6, IL-8) and mucin genes (mucin 5AC/5B) induced by e-cigarette in BAL fluid and lung tissue of experimental mice.
Materials and MethodsMaterialsWe used eGo ONE CT (Shenzhen Joyetech Co., Ltd, ShenZhen, China; battery capacity, 2200 mAh; resistance, 1.0 ohm; air hole, 1.8 mm; atomizer capacity, 2.5 mL; power, 15 W; length, 131.2 mm√Ч19 mm) e-cigarette. The e-cigarette liquid consists of vegetable glycerin, propylene glycol, and liquid nicotine, each of which was purchased from SK Chemicals (Seoul, Korea), LG Household & Health Care (Ulsan, Korea), and VAPER TOWN (Saint Louis, MO, USA) respectively. E-cigarette liquid was made by adding 24 mg/mL of nicotine into the propylene glycol:vegetable glycerin 5:5 ratio solution. LPSs and dexamethasone were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Mucin 5AC (ab3649) and mucin 5B (ab87376) primary antibodies were purchased from Abcam (Cambridge, England, UK). IL-1ќ≤ from Cell Signaling Technology (Danvers, MA, USA), IL-6 from Abcam (Cambridge, England, UK), IL-8 from Thermo Fisher Scientific Inc. (Boston, MA, USA), and HRP-conjugated secondary antibody from NOVUS Biologicals (Centennial, CO, USA) were used. KRG and saponin were provided by Korea Ginseng Corporation (KGC, Daejeon, Korea).
Animal and husbandaryWild-type BALB/c mice (5-6 weeks old; male; weight, 15-18 g) from Koatech (Pyeongtaek, Korea) are used after 7 days acclimation period. Experimental mice were allowed to freely drink water and feed with constant temperature (20-25°C) and humidity (40%-45%). Mice were treated following the guidelines for the care of laboratory animals issued by Yeungnam University. All of the experimental protocols were approved by the Ethical Committee of Yeungnam University (YUMC-AEC2020-012).
LPS and e-cigarette vapor exposure and treatmentLPS was injected to mice intraperitoneally to induce acute inflammation once a day for 2 days. Mice were exposed to e-cigarette vapor made by 40 times puff using a smoking device. The exposure was twice a day for 30 minutes for 28 days. KRG (5 or 10 mg/mL), saponin (50 or 100 ќЉg/mL), and dexamethasone (10 mg/kg) dissolved in phosphate buffer saline (PBS) were administered by oral feeding from the 18th day after exposure to e-cigarette smoke for 10 days.
BAL and lung tissue preparationKetamine (100 mg/kg) and xylazine (10 mg/kg) were injected intraperitoneally for anesthesia in mice for BAL. A cannula was inserted into the trachea, and 1 mL PBS was slowly injected and withdrawn to obtain a BAL solution. The sample was subjected to centrifugation at 400 times gravity at 4°C. The supernatant was preserved by freezing it at 80°C for subsequent analysis. The pellet was diluted with PBS and counted using a hemocytometer. The lung tissue was collected by incision in the chest of mice in the same anesthesia.
Real-time polymerase chain reactionReal-time polymerase chain reaction (PCR) was carried out with GeneAmp RNA core kit and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) using T100 Thermal Cycler and CFX96 real-time PCR system C1000 Thermal Cycler (Bio-Rad). Primers for muicn 5AC (QT01329615; Qiagen, Hilden, Germary) and mucin 5B (10025636, qHsaCIP0028135; Bio-Rad) were used. The primary sequences of the primers used in this experiments are as follows (Table 1). The collected lung tissue was dirsrupted by tissue homogenizer (FastPrep-24; MP Biomedicals, Strasbourg, France), washed 3 times with PBS containing 2% bovine serum albumin (BSA). And then Trizol (Molecular Research Center, Cincinnati, OH, USA) was used to extract total messenger RNA (mRNA). After synthesizing complementary DNA by performing reverse transcription PCR, real-time PCR was performed. Using iQ SYBR Green Supermix, the process of denaturation at 95°C for 15 seconds and binding reaction at 60°C for 45 seconds was repeated 50 times. The amplification accuracy was evaluated using a melting curve (Roche Applied Science, Mannheim, Germany).
Enzyme linked immunosorbent assayThe proteins were extracted from the lung tissues disrupted by tissue homogenizer with radioimmunoprecipitation assay buffer (Thermo Fisher Scientific Inc., Rockford, IL, USA) and quantitatively analyzed using bicinchoninic acid. 20 ќЉg of the extracted proteins and the BAL supernatant were incubated in F96 Cert. Maxisorp Nunc-Immuno plate (Fisher Scientific, Lenexa, KS, USA) for 24 h at 4¬∞C. The samples were first incubated with a 2% BSA solution for 1 hour. Afterward, they were washed 3 times with PBS and then exposed to primary antibodies against IL-1ќ≤, IL-6, IL-8, and MUC5AC/5B. The primary antibodies were diluted at a 1:200 with PBS supplemented with 0.05% Tween 20. Further, the horseradish peroxidase (HRP)-conjugated secondary antibody was diluted 1:5000 in PBS containing 0.05% Tween 20 and added to each well. After 1 h, color was developed with 3,3вАЩ,5,5вАЩ-tetramethylbenzidine peroxidase solution, and the reaction was stopped with 2N-H2SO4. Absorbance measurement was done with EL800 enzyme linked immunosorbent assay reader (BioTek Instruments, Winooski, VT, USA). The protein quantity was determined using a standard curve.
StainingsDiff-Quick (DQ) (Sysmex, Tokyo, Japan) stain was done and cell differentiation was counted based on cell morphology. Hematoxylin and eosin (H&E) stain was performed with the H&E staining kit (Abcam, Cambridge, England, UK) and confirmed with a microscope. Periodic acid-Schiff (PAS) staining was done using a PAS staining kit (Sigma-Aldrich, Saint Louis, MO, USA), and checked under a microscope. Immunohistochemistry (IHC) staining was done in paraffin-embedded lung tissues of mice. According to HRP conjugated secondary antibody method, we checked the protein of MUC5AC/5B. Color development was done using the DAB substrate kit (Abcam), and the slides were counterstained with hematoxylin (Dako, Carpinteria, CA, USA). Then, the slide glass was attached, and it was checked at 200 times using a Nikon fluorescence microscope (Ti-S, 733551; Nikon, Tokyo, Japan).
Statistical analysisFor statistical analysis, SPSS version 22.0 for Windows (IBM Corp., Armonk, NY, USA) was used. All experiments were performed 3 or more times. Data was expressed as the means±standard deviation. We used the Mann-Whitney U test for comparisons. Statistical significance was set when p value was less than 0.05.
ResultsE-cigarette induced the inflammation in airway epithelium of miceInflammatory cells are increased in BAL fluid of mice exposed to e-cigarette and LPS in DQ stain. Inflammatory cells are significantly increased in lung tissue of mice exposed to e-cigarette and LPS in PAS, H&E stain (Fig. 1). E-cigarette caused the acute inflammation in airway epithelium of mice similar to LPS.
E-cigarette induced the expression of cytokines in airway epithelium of miceE-cigarette significantly increased the cytokines (IL-1ќ≤, IL-6, IL-8) mRNA expression and protein production similar to LPS in lung tissue of mice (Fig. 2).
KRG and saponin decreased the expression of cytokines induced by e-cigarette in airway epithelium of miceTo investigate the effects of KRG and saponin on cytokines induced by e-cigarette, mice were made to ingest KRG (5 or 10 mg/mL), saponin (50 or 100 ќЉg/mL), and dexamethasone (10 mg/kg) for 10 days. KRG and saponin statistically significantly inhibited the protein production of cytokines (IL-1ќ≤, IL-6, IL-8) induced by e-cigarette in lung tissue of mice (Fig. 4). Dexamethasone (10 mg/kg) presented the similar results.
KRG and saponin decreased the expression of proteins induced by e-cigarette in airway epithelium of miceTo identify the effects of KRG and saponin on cytokines induced by e-cigarette, mice were made to ingest KRG (5 or 10 mg/mL), saponin (50 or 100 ќЉg/mL), and dexamethasone (10 mg/kg) dissolved in PBS for 10 days. KRG and saponin significantly inhibited the mRNA expression and protein production of MUC5AC/5B induced by e-cigarette in lung tissue of mice (Figs. 5 and 6). In addition, IHC staining confirmed that KRG and saponin suppressed the protein production of MUC5AC/5B increased by e-cigarette (Figs. 5 and 6). Dexamethasone (10 mg/kg) showed the similar results.
DiscussionsAs the use of e-cigarette increases, the side effects of e-cigarette in the respiratory mucous membrane are constantly being identified [1,2]. As the paper on cytokine and chemokine reveals, IL-1ќ≤, IL-6 and IL-8 act as the most important proinflammatory cytokines [7]. MUC5AC/5B, the main components among the various mucin genes, are essential for determining mucociliary clearance and viscoelasticity. Some studies have reported that the expression of MUC5AC increased in patients smoking e-cigarette [22,23]. A previous in vivo study by our research team confirmed that the expression of cytokine and mucin was increased by glyoxal and methylglyoxal, the components of e-cigarette, in human nasal epithelial cells [24]. Furthermore, in this study, it was confirmed that the expression of cytokine and mucin was increased when the vapor of e-cigarette was given to mice in the in vitro study.
The adverse effects of e-cigarette on respiratory epithelial cells were confirmed. Scott, et al. [3] showed that excessive production of reactive oxygen species, inflammatory cytokines and chemokines induced by e-cigarette vapor made alveolar macrophage inflamed and inhibited the phagocytosis. Wu, et al. [4] revealed that e-cigarette increased inflammation and viral infections in human airway epithelial cells. Martin, et al. [5] insisted that e-cigarette activate inflammatory reactions similar to conventional cigarette and suppress immune responses, causing tissue damage in respiratory mucous membranes and making them vulnerable to infection. In this study, we ensured that e-cigarette decreased immune function and overactivated inflammatory reactions like LPS through comparison of inflammatory cells, cytokine, and mucin from BAL fluid and lung tissue of mice. As LPS and e-cigarette showed similar inflammatory reactions in respiratory epithelial cells, it is thought that signal pathway of e-cigarette is associated with TLR-4, TRIF, MyD88, and NF-ќЇB like LPS signal pathway.
KRG is helpful in preventing, controlling, and treating respiratory diseases. Lee, et al. [18] clinically demonstrated the preventive effects of KRG in acute respiratory illness. Also, KRG and saponin showed anti-inflammatory effects and therapeutic potential in chronic respiratory illnesses such as chronic obstructive pulmonary disease and asthma [19,20]. A recent clinical review reported the promising role of KRG against respiratory tract infections [21]. Previously, several researches have confirmed an increase in MUC5AC/5B due to pro-inflammatory cytokines induced by diesel exhaust particle, severe acute respiratory syndrome coronavirus 2, and other factors. In contrast, our study specifically verified the induction of pro-inflammatory cytokines and MUC5AC/5B by e-cigarettes. The significance of our research lies in demonstrating that KRG and saponin can modulate these responses, distinguishing it from earlier studies. The modulation of respiratory inflammatory responses by KRG and saponin was confirmed to be associated with NF-ќЇB pathway [9,10,15,17]. Consequently, we hypothesize that the role of regulating inflammation induced by e-cigarette is also likely to associated with NF-ќЇB pathway. We are currently conducting ongoing research to explore this association. It is believed that respiratory inflammatory reactions stimulated by unavoidable air pollutants or coronavirus can be controlled through KRG and saponin [8,21,25,26]. Additionally, it will be interesting how the respiratory mucosa reacts when people smoke cigarette including KRG as an ingredient.
The main administration route for KRG in humans is oral, so we conducted experiments in a state most similar to that by administering it orally. We chose the safest oral administration method as injecting would not allow us to distinguish whether the inflammatory response observed was due to ecigarette use or not. In a previous study conducted by our research team, we performed a cell viability test, confirming that both low and high doses of KRG and saponin are safe [9,10]. To investigate whether the regulation of inflammatory responses occurs more prominently in proportion to the dose, we compared low and high doses in experiments. However, we did not observe a proportional decrease in effects with dose, and similar regulatory effects were observed at both dosage levels. Recent studies indicate that the typical dosage of KRG used in humans is 0.5-3.0 g/day, and saponin is confirmed at 50-100 mg/day [27]. Through additional research, it is necessary to find an appropriate dosage that combines stability and anti-inflammatory effects in the human body.
There is a limitation in our study. Signal pathway for both e-cigarette and KRG is not accurately identified. We only show the result of effects of KRG and saponin on airway epithelium stimulated by e-cigarette with guessing possible mechanisms. Additional research is needed on which mechanisms e-cigarette cause inflammatory reactions, and KRG and saponin reduce mucin and cytokine in respiratory epithelial cells induced by e-cigarette.
In conclusion, e-cigarette cause the inflammation similar to LPS. KRG and saponin decreased the e-cigarette-induced expression of cytokines and MUC5AC/5B in BAL fluid and lung tissue of mice. KRG and saponin can regulate inflammatory responses induced by e-cigarette in respiratory epithelial cells.
ACKNOWLEDGMENTSThis work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future planning (NRF-2018R1A2B6007756 to YDK).
NotesAuthor Contribution Conceptualization: Sang Jae Lee, Yong-Dae Kim. Data curation: Sang Jae Lee, Yong-Dae Kim. Formal analysis: Hyung Gyun Na. Funding acquisition: Yong-Dae Kim. Investigation: Si-Youn Song. Methodology: Hyung Gyun Na, Yoon Seok Choi. Project administration: Sang Jae Lee, Yong-Dae Kim. Supervision: Yong-Dae Kim. Visualization: Sang Jae Lee, Si-Youn Song. WritingвАФoriginal draft: Sang Jae Lee. WritingвАФreview & editing: Sang Jae Lee, Yong-Dae Kim. Fig.¬†1.The effects of e-cigarette in respiratory epithelial cells. E-cigarette significantly increased inflammatory cells infiltration similar to LPS in Diff-Quick staining (A), PAS staining (B), and H&E staining (C). Yellow arrows indicates inflammatory cells. e-cigarette, electronic cigarette; DQ, Diff-Quick staining; PAS, Periodic acid-Schiff staining; H&E, hematoxyling and eosin staining. 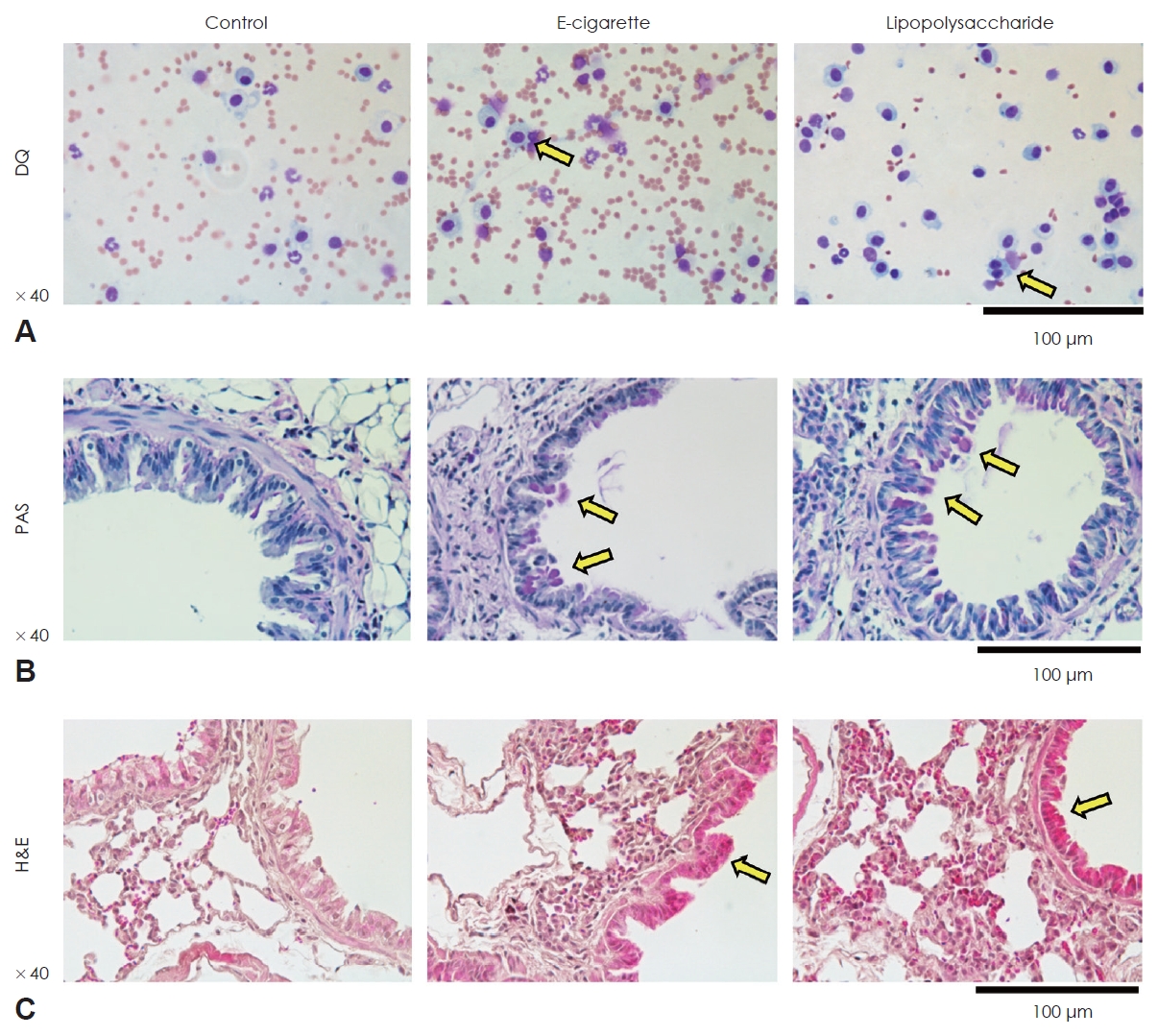
Fig.¬†2.The effects of e-cigarette on cytokines expression in respiratory epithelial cells. E-cigarette significantly increased cytokines (IL-1ќ≤, IL-6, IL-8) mRNA expression (A-C) and protein production (D-F) similar to LPS. Three independent experiments were performed (n=3). Bars represent the mean¬±standard deviation in triplicate. *indicate significance of p<0.05 compared with zero value. Con, control; LPS, lipopolysaccharide; E-cig, e-cigarette; e-cigarette, electronic cigarette; mRNA, messenger RNA. 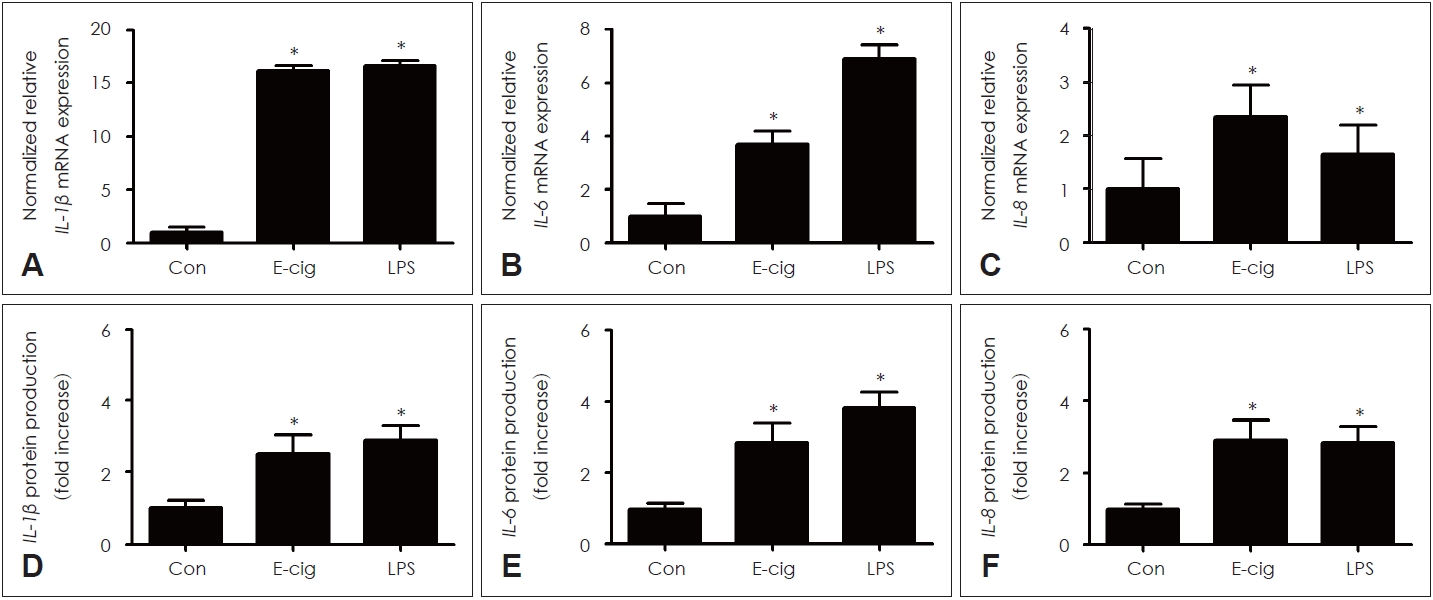
Fig. 3.The effects of e-cigarette on MUC5AC/5B expression in respiratory epithelial cells. E-cigarette significantly increased MUC5AC/5B mRNA expression (A and B) and protein production (C and D) similar to LPS. E-cigarette significantly increased MUC5AC/5B protein production in immunohistochemistry staining (E and F). Three independent experiments were performed (n=3). Bars represent the mean ± standard deviation in triplicate. *indicate significance of p<0.05 compared with zero value. Con, control; LPS, lipopolysaccharide; E-cig, e-cigarette; e-cigarette, electronic cigarette; mRNA, messenger RNA. 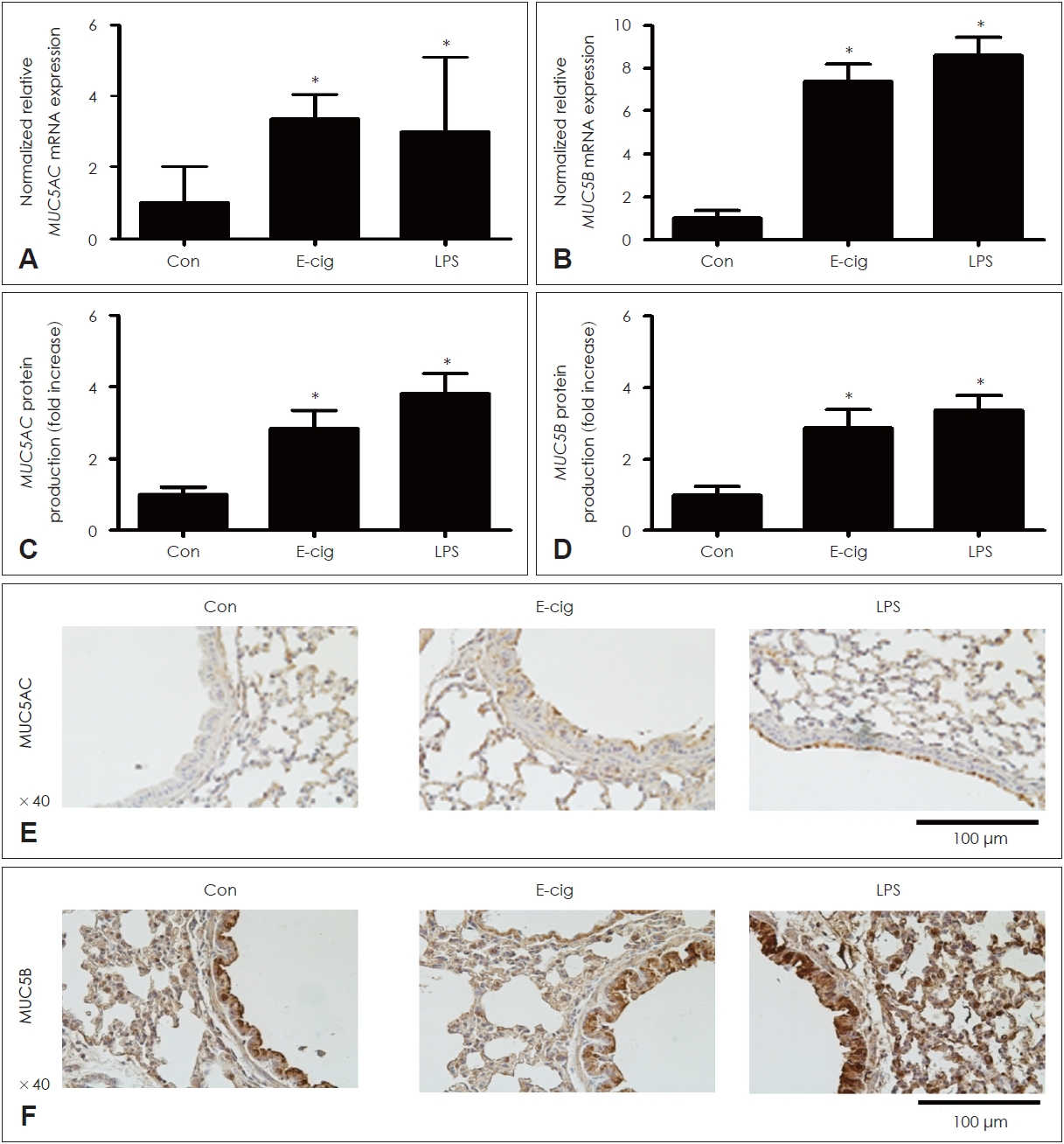
Fig.¬†4.The effects of KRG and saponin on e-cigarette-induced cytokines expression in respiratory epithelial cells. KRG (5 or 10 mg/mL) and saponin (50 or 100 ќЉg/mL) significantly decreased the e-cigarette-induced IL-1ќ≤ (A), IL-6 (B), and IL-8 (C) protein production. Dexamethasone (10 mg/kg) showed the similar effect. Three independent experiments were performed (n=3). Bars represent the mean¬± standard deviation in triplicate. *indicate significance of p<0.05 compared with zero value; вА†indicate significance of p<0.05 compared with e-cigarette only. Con, control; KRG, Korean red ginseng; Sp, saponin; E-cig, e-cigarette; e-cigarette, electronic cigarette; Dexa, dexamethasone; L, low dose; H, high dose. 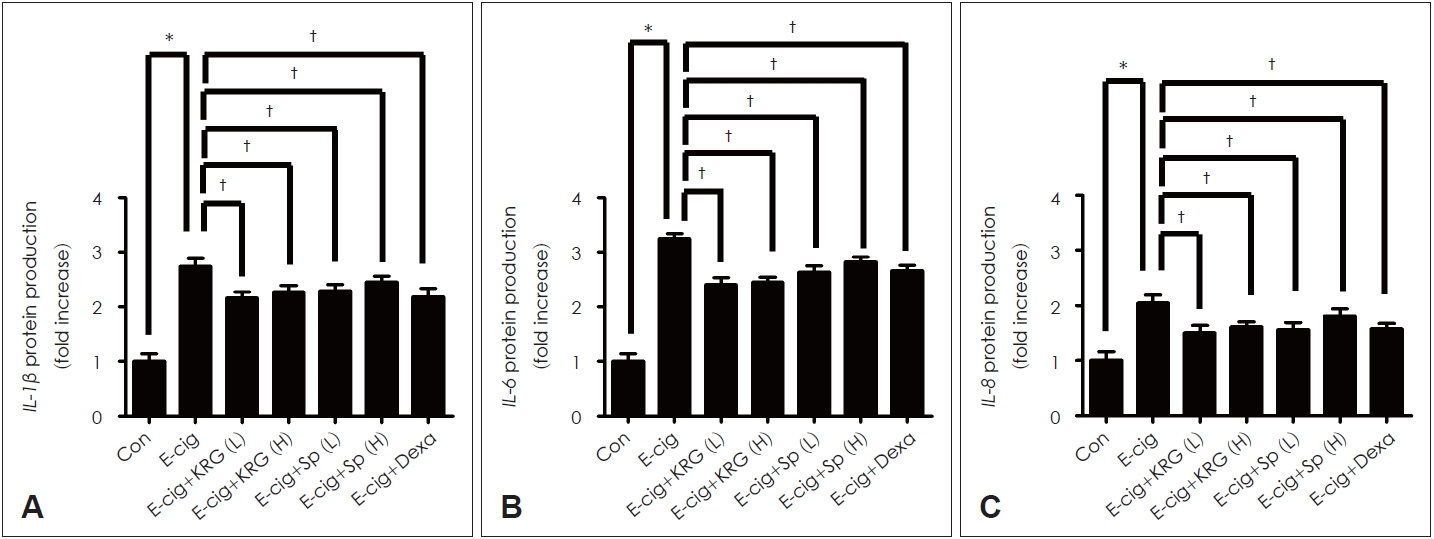
Fig.¬†5.The effects of KRG and saponin on e-cigarette-induced MUC5AC expression in respiratory epithelial cells. KRG (5 or 10 mg/mL) and saponin (50 or 100 ќЉg/mL) significantly decreased the e-cigarette-induced MUC5AC mRNA expression (A) and protein production in enzyme linked immunosorbent assay (B) and immunohistochemistry staining (C). Dexamethasone (10 mg/kg) showed the similar effect. Three independent experiments were performed (n=3). Bars represent the mean¬±standard deviation in triplicate. *indicate significance of p<0.05 compared with zero value; вА†indicate significance of p<0.05 compared with e-cigarette only. Con, control; KRG, Korean red ginseng; Sp, saponin; E-cig, e-cigarette; e-cigarette, electronic cigarette; Dexa, dexamethasone; L, low dose; H, high dose; mRNA, messenger RNA. 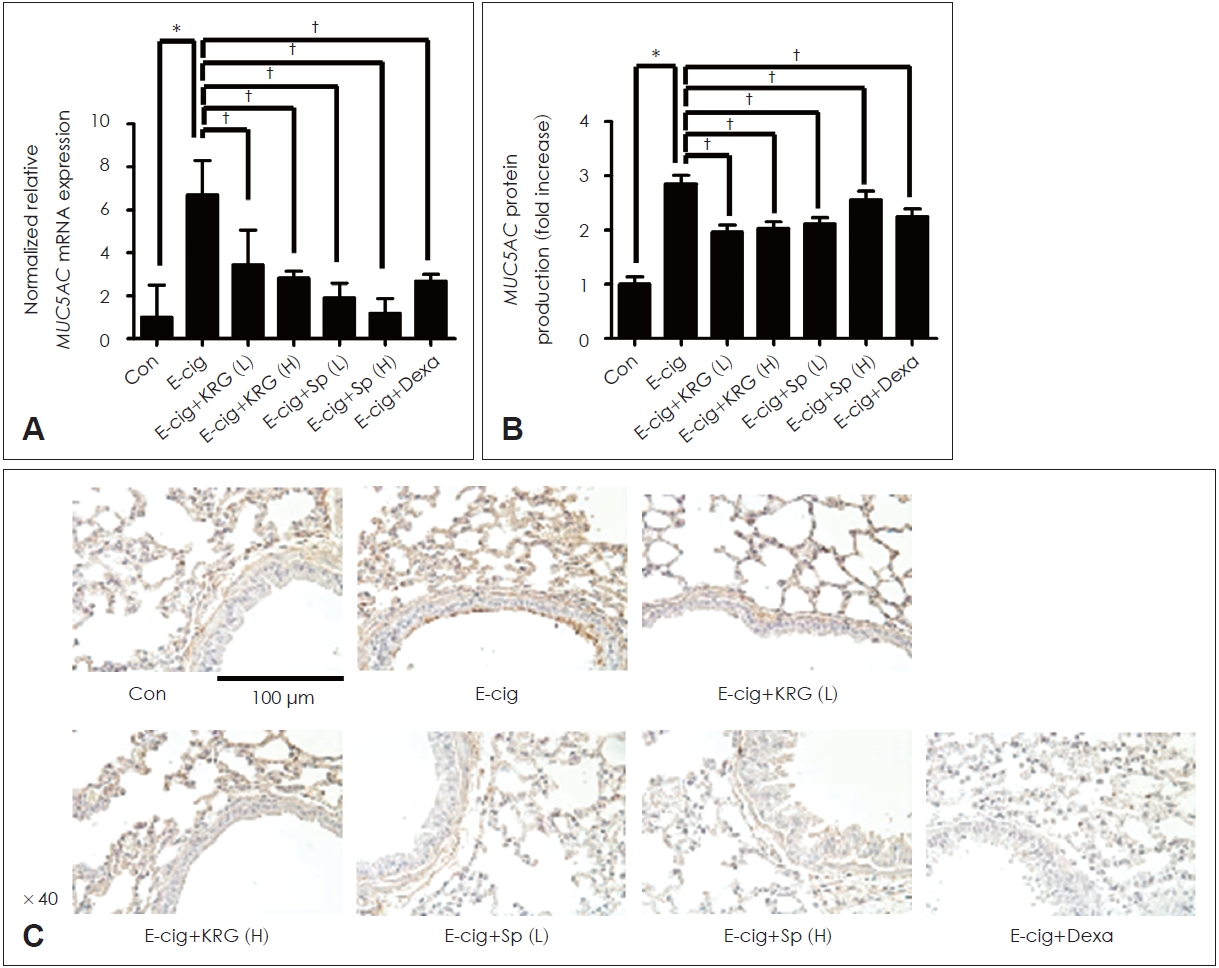
Fig.¬†6.The effects of KRG and saponin on e-cigarette induced MUC5B expression in respiratory epithelial cells. KRG (5 or 10 mg/mL) and saponin (50 or 100 ќЉg/mL) significantly decreased the e-cigarette-induced MUC5B mRNA expression (A) and protein production in enzyme linked immunosorbent assay (B) and immunohistochemistry staining (C). Dexamethasone (10 mg/kg) showed the similar effect. Three independent experiments were performed (n=3). Bars represent the mean¬±standard deviation in triplicate. *indicate significance of p<0.05 compared with zero value; вА†indicate significance of p<0.05 compared with e-cigarette only. Con, control; KRG, Korean red ginseng; Sp, saponin; E-cig, e-cigarette; e-cigarette, electronic cigarette; Dexa, dexamethasone; L, low dose; H, high dose; mRNA, messenger RNA. 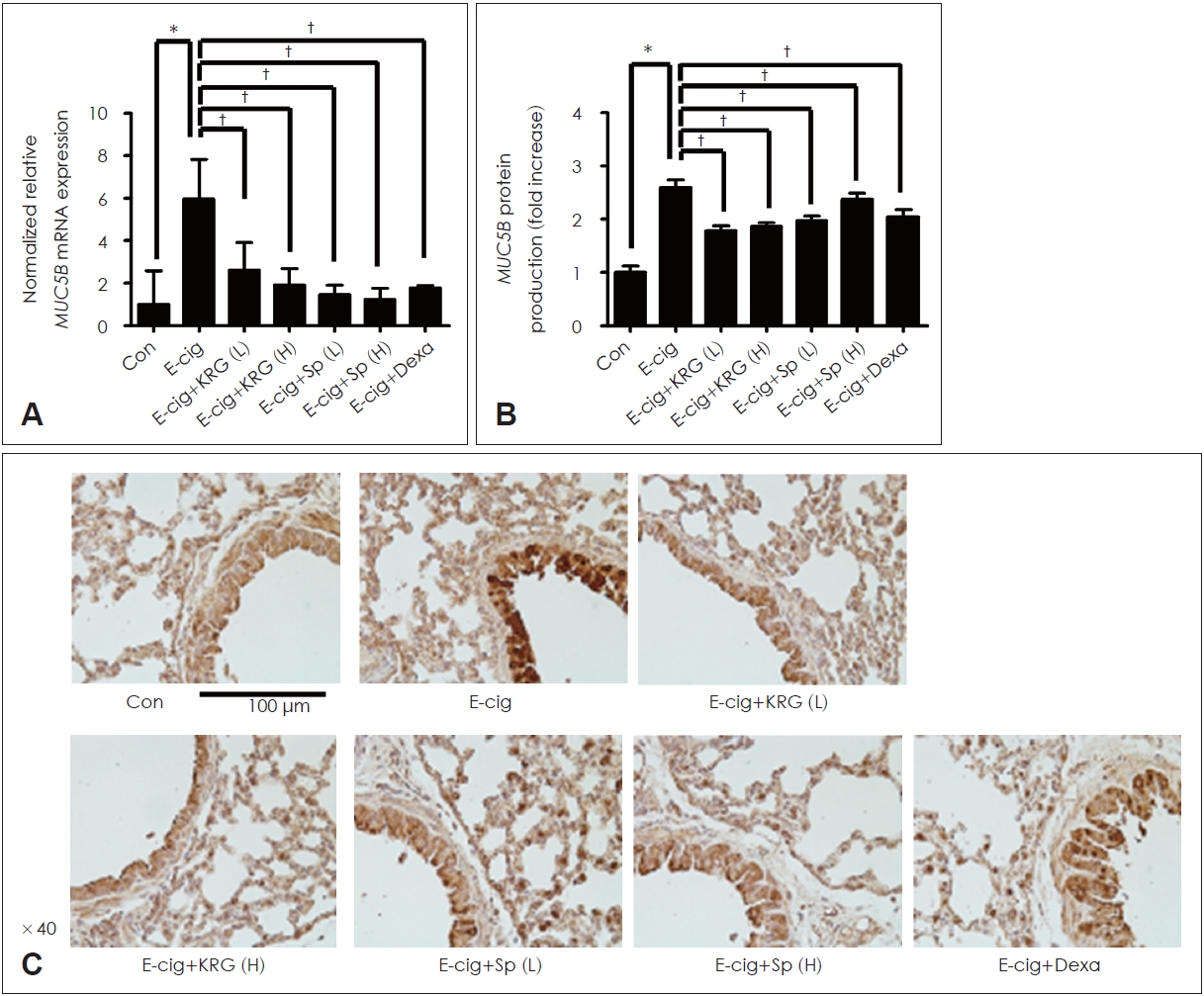
Table 1.Primer used for polymerase chain reaction REFERENCES1. Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes? BMJ 2019;366:l5275.
2. Dinakar C, OвАЩConnor GT. The health effects of electronic cigarettes. N Engl J Med 2016;375(14):1372-81.
3. Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 2018;73(12):1161-9.
4. Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One 2014;9(9):e108342.
5. Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016;311(1):L135-44.
6. Bonser LR, Erle DJ. Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med 2017;6(12):112.
7. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 2014;1843(11):2563-82.
8. Lee S, Na HG, Choi YS, Bae CH, Song SY, Kim YD. SARS-CoV-2 induces expression of cytokine and MUC5AC/5B in human nasal epithelial cell through ACE 2 receptor. Biomed Res Int 2022;2022:2743046.
9. Jo S, Na HG, Choi YS, Bae CH, Song SY, Kim YD. Saponin attenuates diesel exhaust particle (DEP)-induced MUC5AC expression and pro-inflammatory cytokine upregulation via TLR4/TRIF/NF-ќЇB signaling pathway in airway epithelium and ovalbumin (OVA)-sensitized mice. J Ginseng Res 2022;46(6):801-8.
10. Kim YD, Kwak S, Na HG, Choi YS, Bae CH, Song SY. Korean red ginseng inhibits diesel exhaust particles-induced MUC5AC/B expression and inflammation through suppressing TLR4 and NFkB signaling pathway in vitro and in vivo. Eur Respir J 2019;54:PA2406.
11. Lee JO, Yang Y, Tao Y, Yi YS, Cho JY. Korean red ginseng saponin fraction exerts anti-inflammatory effects by targeting the NF-ќЇB and AP-1 pathways. J Ginseng Res 2022;46(3):489-95.
12. Baek KS, Yi YS, Son YJ, Yoo S, Sung NY, Kim Y, et al. In vitro and in vivo anti-inflammatory activities of Korean red ginseng-derived components. J Ginseng Res 2016;40(4):437-44.
13. Chen T, Li B, Qiu Y, Qiu Z, Qu P. Functional mechanism of Ginsenosides on tumor growth and metastasis. Saudi J Biol Sci 2018;25(5):917-22.
14. Shin KK, Yi YS, Kim JK, Kim H, Hossain MA, Kim JH, et al. Korean red ginseng plays an anti-aging role by modulating expression of aging-related genes and immune cell subsets. Molecules 2020;25(7):1492.
15. Kim JH, Yi YS, Kim MY, Cho JY. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res 2017;41(4):435-43.
16. Yi YS. New mechanisms of ginseng saponin-mediated anti-inflammatory action via targeting canonical inflammasome signaling pathways. J Ethnopharmacol 2021;278:114292.
17. Shin SH, Ye MK, Lee DW, Kang BJ, Chae MH. Effect of Korean red ginseng and Rg3 on Asian sand dust-induced MUC5AC, MUC5B, and MUC8 expression in bronchial epithelial cells. Molecules 2021;26(7):2002.
18. Lee CS, Lee JH, Oh M, Choi KM, Jeong MR, Park JD, et al. Preventive effect of Korean red ginseng for acute respiratory illness: a randomized and double-blind clinical trial. J Korean Med Sci 2012;27(12):1472-8.
19. Shergis JL, Di YM, Zhang AL, Vlahos R, Helliwell R, Ye JM, et al. Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement Ther Med 2014;22(5):944-53.
20. Lee IS, Uh I, Kim KS, Kim KH, Park J, Kim Y, et al. Anti-inflammatory effects of ginsenoside Rg3 via NF-ќЇB pathway in A549 cells and human asthmatic lung tissue. J Immunol Res 2016;2016:7521601.
21. Alsayari A, Muhsinah AB, Almaghaslah D, Annadurai S, Wahab S. Pharmacological efficacy of ginseng against respiratory tract infections. Molecules 2021;26(13):4095.
22. Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med 2018;197(4):492-501.
23. Ghosh A, Coakley RC, Mascenik T, Rowell TR, Davis ES, Rogers K, et al. Chronic e-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med 2018;198(1):67-76.
24. Kwak S, Choi YS, Na HG, Bae CH, Song SY, Kim YD. Glyoxal and methylglyoxal as e-cigarette vapor ingredients-induced proinflammatory cytokine and mucins expression in human nasal epithelial cells. Am J Rhinol Allergy 2021;35(2):213-20.
25. Huff RD, Carlsten C, Hirota JA. An update on immunologic mechanisms in the respiratory mucosa in response to air pollutants. J Allergy Clin Immunol 2019;143(6):1989-2001.
|
|
|||||||||||||||||||||||||||||||||||||||

 |
 |