 |
 |
AbstractBackground and Objectives We analyzed the treatment results and prognostic factors of stage IV oral tongue squamous cell carcinoma (OTSCC) patients and explored the existence of subgroups with distinctive prognoses. In addition, the outcome of salvage therapy was analyzed in recurrent cases, and the survival rates and prognostic factors were investigated.
Subjects and Method This study was conducted on patients who were diagnosed with OTSCC and underwent surgery at our hospital between June 2005 and January 2020. A total of 144 patients with stage IV OTSCC was enrolled.
Results A total of 64 recurrences, local (6), regional (21), distant metastasis (33), and locoregional (4), occurred. Seventy-five patients died because of disease progression during the course of study. The 5-year recurrence-free survival rate was 54.5%, and the 5-year disease-specific survival rate was 49.2%. Surgical margins, lymphovascular invasion (LVI), T classification, and lymph nodes (LNs) metastasis exhibited significant correlation with mortality. LVI and advanced T were statistically important factors for predicting distant metastasis. The treatment outcome of the T4N0 patient group without LN metastasis fared the best, while the treatment outcome of the T4N1-3 patient group with advanced T and N findings was the worst.
Conclusion The major type of treatment failure in stage IV OTSCC patients was distant metastasis, and the related predictors of distant metastasis were LVI and advanced T. In the stage IV OTSCC patient group, there were subgroups with distinct prognosis according to the combination of T and N classification. The T4N0 group had the best survival rate, and the T4N1-3 group had the worst prognosis.
IntroductionOral tongue squamous cell carcinoma (OTSCC) is one of the most common malignant tumors of the oral cavity and occurs mainly after the age of 40 years [1,2]. The staging of OTSCC is performed using the tumor node metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC). According to TNM staging, 24%-50% of OTSCC patients initially are diagnosed with local-regionally advanced (stages IVA and IVB) disease, and the 5-year overall survival rate for these patients has been reported to be 28%-58% [3,4]. Surgical treatment is the preferred initial treatment of stage IV OTSCC treatment, and adjuvant therapy should be considered to prevent disease progression and recurrence [5,6]. The treatment modalities for OTSCC patients are mainly determined based on the TNM stage, but the existing TNM staging system has limitations in accurately predicting the prognoses of OTSCC patients.
In the revised 8th edition of the AJCC staging system, extranodal extension (ENE) was newly reflected in the N classification; if metastatic lymph nodes (LNs) were present with ENE, the patient was upstaged to N2a or N3b. Therefore, cases defined as N1 in the previous 7th TNM system were upgraded to N2a or N3b and newly classified as stage IV. As these patients were reclassified as stage IV, the likelihood of subgroups with distinct prognostic differences among stage IV OTSCC group increased. Therefore, we investigated the treatment results and prognostic factors of stage IV OTSCC patients and explored the existence of subgroups with distinctive prognoses. In addition, the outcome of salvage therapy was analyzed in recurrent cases, and the survival rates and prognostic factors were investigated.
Subjects and MethodsFrom June 2005 to January 2020, we enrolled patients diagnosed with OTSCC and who underwent treatment at Severance Hospital. The Institutional Review Board of Yonsei University approved this retrospective study (IRB No. 3-2024-0140). The 8th AJCC staging system was used for tumor staging of all patients. The inclusion criteria were 1) histological diagnosis of OTSCC and surgery as an initial treatment and 2) pathologic TNM stage IV. Exclusion criteria were as follows: 1) previous surgery or radiotherapy in the head and neck region, 2) stage I-III OTSCC, and 3) distant metastases at the time of diagnosis.
Patient age, gender, smoking history, and other demographics were analyzed based on medical records. Pathological information including histologic grade, surgical margin status, lymphovascular invasion (LVI), perineural invasion (PNI), ENE, tumor size, and cases and causes of death during the study were additionally investigated. Recurrence cases were also checked and classified specifically as follows: local, regional, distant. The period from initial treatment to recurrence was defined as the period to recurrence. Five-year recurrence-free survival (RFS) and disease-specific survival (DSS) were analyzed based on recurrence and mortality analysis data.
All patients included in this study underwent surgery with curative intention. Depending on the size and location of the primary tumor, subtotal or total glossectomy was performed. For cN0, elective neck dissection including level I-III was performed; for cN1, therapeutic neck dissection was performed. Adjuvant therapy was considered if positive/close margins, LVI, PNI, ENE, or T3-4 findings were found in the pathological examination.
Data such as patients’ personal/clinical information, postoperative pathology records, Among the above data, uni/multivariate analyzes were used to determine which factors influenced the survival rate of patients. Statistical techniques such as chi-square test, Fisher’s exact test, and t-test were used. Additionally, the Kaplan-Meier curve and multivariate cox proportional hazard regression model were also used. A p-value <0.05 was considered to indicate statistical significance. Statistical analysis was performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
ResultsA total of 144 patients with stage IV OTSCC was included in this study, with 91 (63.2%) males and 53 (36.8%) females. The mean age of the patients was 52.1 years (range, 18-85 years). According to pT classification, 13 patients were pT1 (9.0%), 44 were pT2 (30.6%), 26 were pT3 (18.1%), and 61 were pT4 (42.4%). According to pN classification, 13 patients were pN0 (9.0%), 8 were pN1 (5.6%), 51 were pN2 (35.4%), and 72 were pN3 (50.0%). The degree of histologic differentiation was evaluated in the surgical specimens. Forty-seven (32.6%) cases were well-differentiated, 50 (34.7%) cases moderately differentiated, 7 (4.9%) cases were poorly differentiated, and 40 (27.8%) cases were unknown. In addition, 127 cases (88.2%) exhibited negative surgical margins and 17 cases (11.8%) showed positive margins. There were 38 cases (26.4%) with LVI findings and 68 cases (47.2%) with PNI findings. ENE findings were present in 85 patients (59.5%). Adjuvant therapy was conducted to 129 patients (89.6%), 48 patients received radiotherapy only, and the rest of patients received CCRT. Sixty-four recurrences occurred during the study period - local (6), regional (21), distant (33), and locoregional (4) each. Seventy-five patients died due to progression during the study period. The 5-year RFS of the patients was 54.5%, and the 5-year DSS was 49.2% (Fig. 1). Table 1 summarizes the other clinical information of all the patients.
When prognostic factors related to disease recurrence were analyzed through univariate analysis, LVI, T classification, and LNs metastasis exhibited significant correlation with disease recurrence (Table 2). On multivariate analysis, LVI, T classification, and LN metastasis also showed significant correlation with disease recurrence. When prognostic factors related to mortality were analyzed through univariate analysis, surgical margins, LVI, T classification, and LNs metastasis exhibited a statistically significant correlation (Table 3). In multivariate analysis including only variables with p-values less than 0.05, surgical margins, LVI, T classification, and LNs metastasis showed significant correlation with mortality (Table 4).
During the study period, 64 relapses occurred, and surgical treatment was a prognostic factor only in relapsed patients on univariate and multivariate analyses (Tables 5 and 6). In relapsed patients, the 5-year DSS after salvage therapy was only 9.7% (Fig. 2). In stage IV OTSCC patients, the most common site of disease recurrence was distant metastasis, followed by regional recurrence. In univariate analysis, LVI, PNI, surgical margin status, and advanced T were statistically significant as predictors of distant metastasis. When multivariate analysis was performed on these variables, LVI and advanced T were statistically significant factors for predicting distant metastasis (Table 7).
The presence or absence of subgroups with distinct prognoses in the stage IV OTSCC patient group was analyzed. The patients were divided into three groups according to T and N combination. Patients were classified into pT4N0 without LN metastasis, pT1-3N2-3 with small primary tumor but advanced N, and pT4N1-3 with both advanced T and N findings. Five-year RFS and DSS were analyzed in the three groups, and statistically significant differences were identified. The treatment outcome of the T4N0 patient group without LN metastasis was the best, while the treatment outcome of the T4N1-3 patient group with advanced T and N findings was the worst (Fig. 3).
DiscussionFor treatment of stage IV OTSCC, surgery is the preferred initial method, and adjuvant therapy should be performed to prevent disease progression and recurrence [5,6]. The incidence of distant metastasis of oral cancer has been reported to be approximately 10%, which is not high compared to other sites of HNSCC [7,8]. However, as locoregional control of oral cancer patients has improved with development of treatment technologies, distant metastasis is emerging as an important cause of treatment failure [9-12]. This study focused on stage IV OTSCC, 51% of all recurrent cases involved distant metastasis, which was the most common type of treatment failure. Of patients with distant metastasis, 90.9% eventually died, indicating again that salvage therapy is very rarely successful when distant metastasis occurs in OTSCC patients. Therefore, if there is a method for screening high-risk groups and providing early diagnosis before clinical presentation of distant metastases, the survival rate of advanced OTSCC patients may improve. Several researchers have argued that chest CT every 6 months for the first 2 years is helpful for early detection in high-risk HNSCC patients because many distant metastases occur in the lungs [13]. LVI findings of tumor cell invasion of blood or lymphatic vessels are important in the tumor metastatic cascade or angiogenesis [9]. In our study, LVI findings along with advanced T were significant predictors of distant metastasis, and it is necessary to perform active surveillance for early detection of distant metastases in high-risk patients with these findings. A previous study reported that adjuvant chemotherapy can be effective in improving survival and reducing distant metastasis in loco-regionally advanced HNSCC patients after surgery and adjuvant radiotherapy [14,15]. Recently, clinical trials on maintenance adjuvant therapy using targeted agents including cetuximab or immune check point inhibitors have been conducted to control distant metastasis in HNSCC patients [16-18]. Further studies are needed to elucidate the clinical effects of aforementioned maintenance adjuvant therapy in preventing distant metastasis and to explore the optimal indications for the treatment in high-risk patients.
It has been known that 40% of oral cancer patients are diagnosed as stage IV initially, and the 5-year survival rate of these patients is 28%-58% [19-25]. In the 8th AJCC staging system, stage IV corresponds to cases with advanced T (T4a or T4b) or aggressive LNs metastasis (N2-3). Therefore, a case of T4 without LN metastasis or a case of N2 or N3 regardless of T classification also is classified as stage IV. Accordingly, the stage IV OTSCC patient group can be divided into several subgroups according to combination of T and N classification. In this study, stage IV OTSCC patients were divided into T4N0, T1-3N2-3, and T4N1-3, and 5-year RFS of these three patient groups was 84.6%, 56.4%, and 41.4%, respectively, exhibiting a statistically significant difference. In addition, the 5-year DSS of the three patient groups was 76.9%, 52.9%, and 35.0%, respectively, which also showed a statistically significant difference. Most of the patients participating in this study were treated with the same treatment protocol, including surgery and adjuvant therapy (radiotherapy or chemoradiotherapy), but demonstrated different levels of tumor control for each subgroup. Although it is not considered in the current standard of care, it can be seen that active follow-up strategies or more intensified adjuvant therapy may be needed for early diagnosis or prevention of disease recurrence in the T1-3N2-3 and T4N1-3 patient groups with a poorer prognosis.
Since current TNM staging does not reflect important pathological factors such as LVI, PNI, and surgical margins, it has limitations in predicting the prognosis of patients. As shown in this study, LVI and surgical margin status in stage IV OTSCC are important prognostic factors related to the survival of patients. Therefore, these factors should be reflected in the risk stratification of the patient group. Some researchers have argued that the survival rate of patients can be improved by devising a nomogram that can predict the treatment outcome of oral cancer patients and suggesting intensified adjuvant treatment for high-risk patients [26]. In the future, additional research is needed to classify stage IV OTSCC patients according to individualized risk using methods such as nomograms and to improve the survival rate of these patients by selecting high-risk groups.
Since this study was conducted retrospectively, selection bias related to patient selection cannot be avoided. In addition, since T4b patients were not included, there is a limit to its application for interpretation of treatment results for the T4b patient group. Since this study was conducted focused on the data of patients who underwent surgery and adjuvant radiation or chemotherapy, it has limitations in its application to patients who underwent curative chemotherapy. In addition, there is a limitation in applying the results of this study to these patients as it does not reflect the treatment protocols, including neoadjuvant chemotherapy and surgery, which are being implemented in some institutions.
In conclusion, the major type of treatment failure in stage IV OTSCC patients was distant metastasis, and the related predictors of distant metastasis were LVI and advanced T. In the stage IV OTSCC patient group, there were subgroups with distinct prognoses according to the combination of T and N classifications. The T4N0 group had the best survival rate, while the T4N1-3 group had the worst prognosis.
ACKNOWLEDGMENTSThis work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1C1C1012004).
NotesAuthor contributions Conceptualization: Yoon Woo Koh, Se-Heon Kim, Jae-Yol Lim. Data curation: Moon Su Kwak, Yoon Woo Koh, Se-Heon Kim, Jae-Yol Lim. Methodology: Yoon Woo Koh, Se-Heon Kim, Jae-Yol Lim. Resources: Yoon Woo Koh, Jae-Yol Lim. Supervision: Yoon Woo Koh, Se-Heon Kim, Jae-Yol Lim. Writing—original draft: Dae Hyun Kim, Young Min Park. Writing—review & editing: Moon Su Kwak. Fig. 1.The 5-year recurrence-free survival (RFS) of the patients was 54.5%, while the 5-year disease-specific survival (DSS) was 49.2%. A: 5 yr RFS. B: 5 yr DSS. C: 5 yr DSS of relapsed patients after salvage therapy. 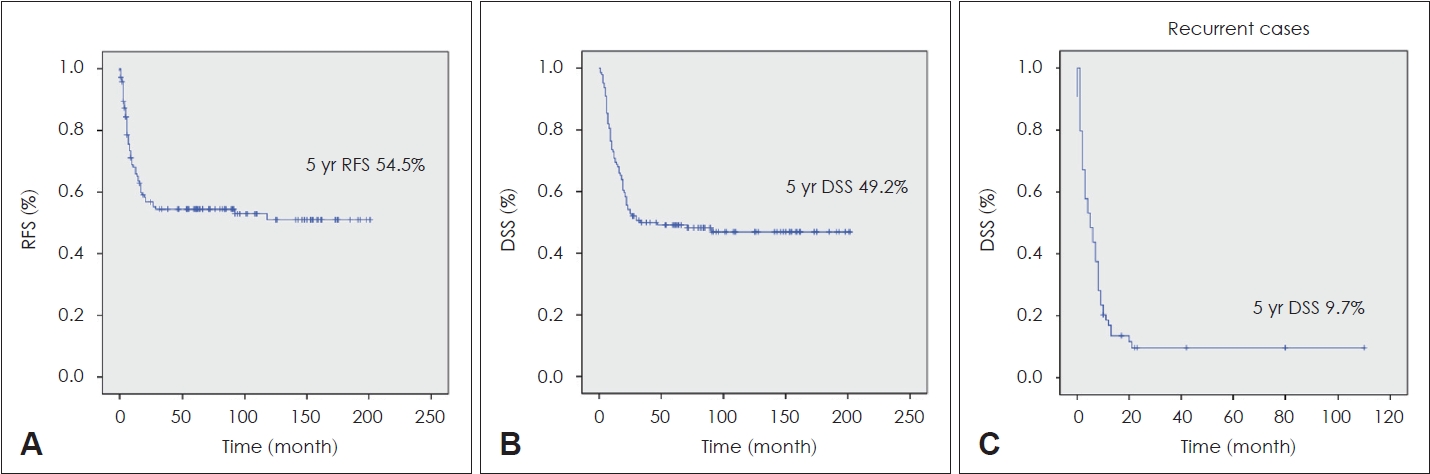
Fig. 2.The 5-year disease-specific survival (DSS) of relapsed patients after salvage therapy was 9.7%. 5 yr DSS of relapsed patients after salvage therapy. 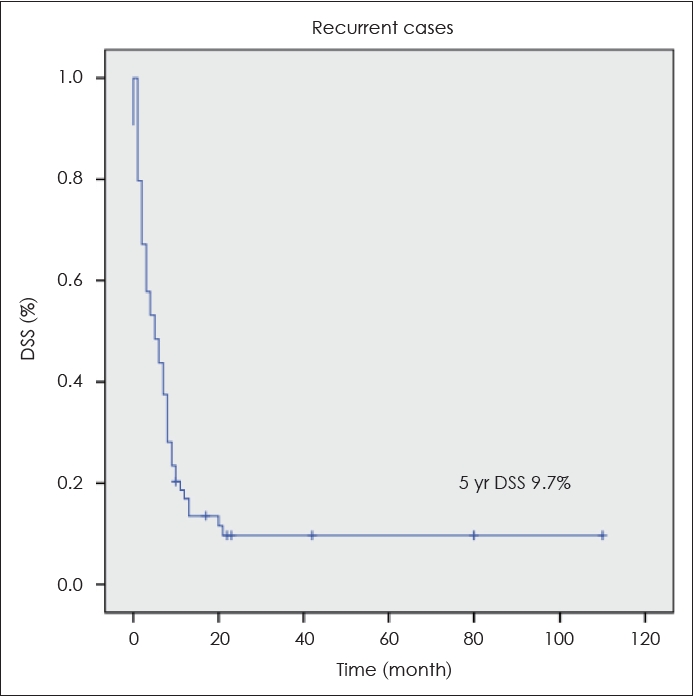
Fig. 3.Subgroup analysis (T4N0 vs. T1-3N2-3 vs. T4N1-3) of 5-year recurrence-free and disease-specific surgical of stage IV tongue cancer patients. A: Subgroup analysis of 5 yr recurrence-free survival (RFS) rate in stage IV tongue cancer patients. B: Subgroup analysis of 5 yr disease-specific survival (DSS) rate in stage IV tongue cancer patients. 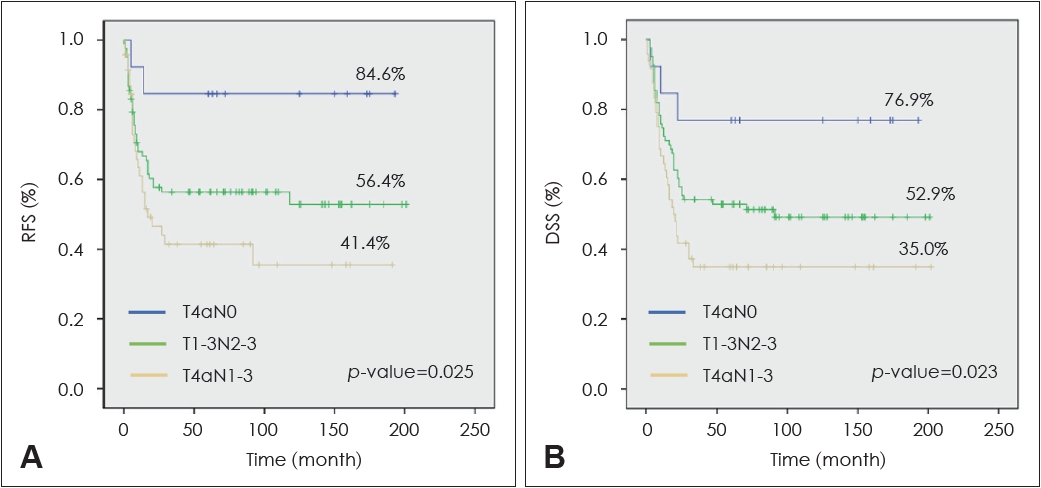
Table 1.Clinical information of all patients (n=144) enrolled in the study Table 2.Univariate analysis of clinic-pathologic factors on 5-year RFS Table 3.Univariate analysis of clinic-pathologic factors on 5-year DSS Table 4.Multivariate Cox regression analysis for RFS and DSS on various factors Table 5.Univariate analysis of variables for predicting survival in recurrent cases
Table 6.Multivariate Cox regression analysis for predicting survival in recurrent cases
REFERENCES1. Sasaki T, Moles DR, Imai Y, Speight PM. Clinico-pathological features of squamous cell carcinoma of the oral cavity in patients <40 years of age. J Oral Pathol Med 2005;34(3):129-33.
2. Bodner L, Manor E, Friger MD, van der Waal I. Oral squamous cell carcinoma in patients twenty years of age or younger--review and analysis of 186 reported cases. Oral Oncol 2014;50(2):84-9.
3. Chitapanarux I, Lorvidhaya V, Sittitrai P, Pattarasakulchai T, Tharavichitkul E, Sriuthaisiriwong P, et al. Oral cavity cancers at a young age: analysis of patient, tumor and treatment characteristics in Chiang Mai University Hospital. Oral Oncol 2006;42(1):83-8.
4. Zhang YY, Wang DC, Su JZ, Jia LF, Peng X, Yu GY. Clinicopathological characteristics and outcomes of squamous cell carcinoma of the tongue in different age groups. Head Neck 2017;39(11):2276-82.
5. Sessions DG, Spector GJ, Lenox J, Haughey B, Chao C, Marks J. Analysis of treatment results for oral tongue cancer. Laryngoscope 2002;112(4):616-25.
6. Fein DA, Mendenhall WM, Parsons JT, McCarty PJ, Stringer SP, Million RR, et al. Carcinoma of the oral tongue: a comparison of results and complications of treatment with radiotherapy and/or surgery. Head Neck 1994;16(4):358-65.
7. Uchiyama Y, Sasai T, Nakatani A, Shimamoto H, Tsujimoto T, Kreiborg S, et al. Distant metastasis from oral cavity-correlation between histopathology results and primary site. Oral Radiol 2021;37(2):167-79.
8. Sumioka S, Sawai NY, Kishino M, Ishihama K, Minami M, Okura M. Risk factors for distant metastasis in squamous cell carcinoma of the oral cavity. J Oral Maxillofac Surg 2013;71(7):1291-7.
9. Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004;5(10):816-26.
10. Barros-Silva PG, Fontes-Borges MM, Costa-Dias C, Mota-Lemos JV, Socorro-Saldanha-Cunha MD, Fernandes-Souza E, et al. Clinical-pathological and sociodemographic factors associated with the distant metastasis and overall survival of oral cavity and oropharynx squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 2020;25(3):e375-82.
11. Aires FT, Lin CS, Matos LL, Kulcsar MAV, Cernea CR. Risk factors for distant metastasis in patients with oral cavity squamous cell carcinoma undergoing surgical treatment. ORL J Otorhinolaryngol Relat Spec 2017;79(6):347-55.
12. Bugshan A, Farooq I. Oral squamous cell carcinoma: metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Res 2020;9:229.
13. Hsu YB, Chu PY, Liu JC, Lan MC, Chang SY, Tsai TL, et al. Role of chest computed tomography in head and neck cancer. Arch Otolaryngol Head Neck Surg 2008;134(10):1050-4.
14. Huang WY, Ho CL, Chao TY, Lee JC, Chen JH. Oral tegafur-uracil as a metronomic therapy in stage IVa and IVb cancer of the oral cavity. Am J Otolaryngol 2021;42(6):103156.
15. Hsieh MY, Chen G, Chang DC, Chien SY, Chen MK. The impact of metronomic adjuvant chemotherapy in patients with advanced oral cancer. Ann Surg Oncol 2018;25(7):2091-7.
16. Addeo R, Montella L, Mastella A, Vincenzi B, Mazzone S, Ricciardiello F, et al. Maintenance therapy with biweekly cetuximab: optimizing schedule can preserve activity and improves compliance in advanced head and neck cancer. Oncology 2018;95(6):353-9.
17. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359(11):1116-27.
18. Machiels JP, Tao Y, Burtness B, Tahara M, Licitra L, Rischin D, et al. Pembrolizumab given concomitantly with chemoradiation and as maintenance therapy for locally advanced head and neck squamous cell carcinoma: KEYNOTE-412. Future Oncol 2020;16(18):1235-43.
19. Patil VM, Prabhash K, Noronha V, Joshi A, Muddu V, Dhumal S, et al. Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers. Oral Oncol 2014;50(10):1000-4.
20. Eder-Czembirek C, Czembirek C, Selzer E. Neoadjuvant radiotherapy plus radical surgery for locally advanced stage III/IV oral cancer: analysis of prognostic factors affecting overall survival. Oral Oncol 2016;60:1-7.
21. Garzino-Demo P, Dell’Acqua A, Dalmasso P, Fasolis M, La Terra Maggiore GM, Ramieri G, et al. Clinicopathological parameters and outcome of 245 patients operated for oral squamous cell carcinoma. J Craniomaxillofac Surg 2006;34(6):344-50.
22. Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY, et al. Surgical outcome of T4a and resected T4b oral cavity cancer. Cancer 2006;107(2):337-44.
23. Lo WL, Kao SY, Chi LY, Wong YK, Chang RC. Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: factors affecting survival. J Oral Maxillofac Surg 2003;61(7):751-8.
24. Rogers SN, Brown JS, Woolgar JA, Lowe D, Magennis P, Shaw RJ, et al. Survival following primary surgery for oral cancer. Oral Oncol 2009;45(3):201-11.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |