Introduction
Invasive fungal rhinosinusitis (IFRS) represents a spectrum of aggressive fungal infections characterized by fungal invasion of the sinonasal mucosa, submucosa, blood vessels, or bones. It predominantly affects immunocompromised patients and carries a high mortality rate despite advances in medical and surgical management. IFRS can be classified into three major types based on the duration of symptoms and histopathological features: acute invasive fungal rhinosinusitis (AIFRS), chronic invasive fungal rhinosinusitis (CIFRS), and chronic granulomatous invasive fungal rhinosinusitis (CGIFRS) [1,2].
AIFRS develops rapidly (within 4 weeks) and predominantly affects severely immunocompromised patients, such as those with hematological malignancies, poorly controlled diabetes mellitus (DM), or recipients of immunosuppressive therapy following organ transplantation or chemotherapy for solid organ malignancies. Mucorales (Mucor, Rhizopus, Rhizomucor) and Aspergillus species are the most common causative organisms [1,2]. The characteristic histopathological finding is the fungal invasion of blood vessels, causing thrombosis and tissue necrosis. Surgical debridement, systemic antifungal therapy, and correcting underlying systemic disease are crucial for treating AIFRS [3-5]. A 2013 review reported a mortality rate of 49.7%, with advanced age and intracranial involvement being poor prognosis factors, while DM and surgical resection were good prognostic factors [6]. However, a recent study using the 2000-2014 National (Nationwide) Inpatient Sample database in the United States showed a lower mortality rate of 15.8%, associating mucormycosis, pneumonia, hematologic disorders, and age (per decade) with higher odds of inpatient mortality, and DM with lower odds [7].
CIFRS presents with symptoms lasting more than 12 weeks and is characterized by a dense accumulation of fungal hyphae with tissue and vascular invasion, leading to necrosis but minimal inflammatory response. CGIFRS, on the other hand, shows noncaseating granulomas with foreign body or Langerhans-type giant cells, vasculitis, and sparse fungal hyphae [1,2]. CIFRS is generally more symptomatic and is associated with a poorer prognosis, occurring primarily in immunocompromised patients, whereas CGIFRS is likely to present more insidiously in immunocompetent patients [8-11].
Recent research has underscored the significance of early diagnosis and aggressive management in enhancing outcomes. This review article aims to provide an updated overview of the clinical features, diagnostic approaches, prognostic factors, and treatment strategies for IFRS (Table 1).
Epidemiology and Risk Factors
IFRS is relatively rare; however, the mortality rate of IFRS remains high, ranging from 15% to 50% despite improvements in medical and surgical management [5,7,9,12].
The most significant risk factor for developing IFRS is an immunocompromised state. Predisposing conditions for IFRS include old age, DM, hematological malignancies, solid organ transplantation, chemotherapy for solid malignancies, and prolonged corticosteroid use. In particular, poorly controlled DM with ketoacidosis, which impairs neutrophil function, and hematological malignancies causing neutropenia were the most common underlying conditions [7,13-15].
AIFRS and CIFRS are more commonly found in immunocompromised and diabetic patients, whereas CGIFRS mainly affects immunocompetent individuals with prominent geographic clustering in subtropical regions. There are no individual exposures definitely associated with CIFRS or CGIFRS. The higher prevalence in countries with hot and dusty climates suggests that exposure to dust and environmental molds may predispose patients to CIFRS or CGIFRS [5,8-11,16]. In our institutional experience, the prevalence of AIFRS was highest, followed by CIFRS and CGIFRS. Most patients with AIFRS, CIFRS, or CGIFRS were diabetic or immunocompromised, and some patients with CIRFS or CGIFRS were immunocompetent and older than 70 years. However, there were no significant differences in risk factors between CIFRS and CGIFRS [15,17].
Clinical Presentation
Symptom variance is related to disease progression, tissue involvement, and the timing of diagnosis [6]. Presentations range from minimal sinonasal symptoms to severe manifestations, including visual impairment, ophthalmoplegia, ptosis, and altered mental status in widespread disease. Early diagnosis of AIFRS requires high suspicion in at-risk patients as initial symptoms can be nonspecific and resemble those of bacterial sinusitis, such as nasal congestion, rhinorrhea, and crusting, which can lead to delayed diagnosis [18,19].
The common symptoms include severe facial pain, headache, nasal congestion, rhinorrhea, fever (which is more common in patients with hematological malignancies), facial swelling, ophthalmoplegia, vision loss, proptosis, and facial numbness or paresthesia [6,13-15,20-24]. Facial pain and headaches are typically not controlled with analgesics [14,15]. A recent multidisciplinary consortium suggested that symptom categories could be grouped into constitutional, sinonasal, otolaryngological, cranial nerve, and ophthalmologic manifestations [5]. Constitutional symptoms observed in patients include fever (present in 9%-100% of cases), headache (present in 25%-100%), and altered mental status (present in 11%-27%) [6,19,22,25-29]. Sinonasal manifestations most frequently include nasal congestion (reported in 14%-100% of cases) and crusting or eschar formation (observed in 8%-90% of cases) [6,24,29-33]. Otolaryngologic features frequently manifest as facial swelling (in 27%-100% of patients) and pain (in 14%-86% of patients) [22,23,30,34-36]. Cranial nerve involvement manifests as facial anesthesia in 8%-55% and facial paralysis in 25%-44% [21,23-25,37,38]. Ophthalmologic complications include visual acuity loss in 26%-87%, proptosis in 16%-100%, and ophthalmoplegia in 17%-60% [27,28,34,36,38-40]. Extrasinonasal extension typically presents with cranial nerve and ophthalmologic symptoms.
Diagnosis
Early diagnosis of IFRS is crucial for improved outcomes. The diagnostic approach includes clinical evaluation, laboratory tests, imaging studies, and histopathological confirmation.
Endoscopic findings
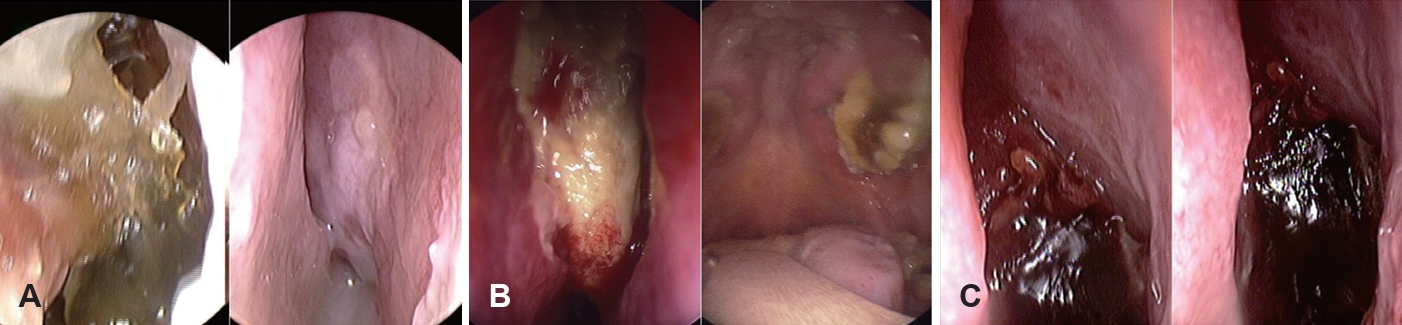
Nasal endoscopy is commonly used to evaluate suspected AIFRS patients by directly visualizing the nasal cavity. Key findings suggestive of AIFRS include mucosal edema, crusting, friability, tissue discoloration (pallor or darkening), ulceration, necrosis, and lack of sensation (Fig. 1). Although the procedure exhibits high specificity for detecting abnormalities, its sensitivity ranges considerably from 49% to 75% [19,23,38,41,42], indicating that negative findings cannot reliably rule out the disease.
Limited research exists on the routine use of endoscopy [43]. Major limitations include the inability to visualize paranasal sinuses in non-operated patients, post-surgical debris interference, and patient intolerance. Endoscopic changes may indicate late-stage disease. Despite these limitations, nasal endoscopy remains valuable due to its low cost, minimal time investment, and high specificity for identifying lesions. While nasal endoscopy has limited sensitivity as a screening tool, reducing its effectiveness for early AIFRS detection, it should be performed in immunocompromised patients if IFRS is suspected to identify mucosal abnormalities.
Laboratory tests
Recently, blood tests for serum fungal biomarkers such as galactomannan (GM) and 1,3-╬▓-D-glucan (BDG) have become widely available and are now used to screen for and diagnose invasive fungal diseases early [37,44]. GM is a polysaccharide antigen found in the cell walls of Aspergillus species, which may be positive in invasive aspergillosis [45]. BDG is a component of the cell walls of many fungi (e.g., Candida, Aspergillus, and Pneumocystis gerovesi), making this test more useful for broadly detecting fungal pathogens than for identifying specific ones. A previous meta-analysis has shown that BDG can accurately distinguish between possible invasive fungal diseases and noninvasive fungal diseases [45,46]. The cross-reactivity of GM with ╬▓-lactam antibiotics is disputed [47-49], and early BDG test can give false positive results for several antibiotics, bacteremia, and malignancy, and is not specific to a fungal species [44,47]. The GM antigen and BDG tests exhibited low sensitivity and positive predictive value (PPV) when used to diagnose IFRS while showing high specificity over 90% and negative predictive value (NPV) [37]. Serum GM antigen was more frequently positive in AIFRS compared to CIFRS and CGIFRS [15].
For suspected IFRS, laboratory evaluation includes a complete blood count to detect neutropenia and blood tests for glucose and HbA1c to assess diabetes and glycemic control. Neutropenia or prolonged neutropenia may be associated with increased mortality, and recovery of absolute neutrophil count may be associated with improved survival [3,6,21,23,26,33,38,40]. A systematic review of 807 patients with AIFRS demonstrated that the prevalence of diabetes was 47.8%, including 23.1% diagnosed with diabetic ketoacidosis. These patients also presented with other risk factors, including hematologic malignancy, corticosteroid treatment, solid-organ transplantation, human immunodeficiency virus/acquired immunodeficiency syndrome, and an autoimmune disease. However, a prognostic analysis completed on 398 patients found that DM was a positive prognostic indicator in both univariate and multivariate analyses as compared with other immunosuppressive diagnoses [6]. Other studies have also reported that well-controlled DM is a positive prognostic factor [7,13]. C-reactive protein has been shown to have conflicting associations with survival, making it difficult to use as a prognostic factor [21,40,50].
Imaging studies
CT is usually the first-line imaging modality for suspected sinusitis. Findings suggestive of IFRS include mucosal thickening, bone erosion or destruction, and soft tissue infiltration into adjacent structures such as the orbit, facial soft tissue, and the retroantral fat pad. However, CT findings can be nonspecific and mimic bacterial sinusitis in the initial stages of IFRS. Thus, CT scans only identify AIFRS once bone erosion or soft tissue infiltration is apparent, making the early diagnosis of IFRS challenging [51-53]. Bone destruction on CT was detected in only 50%-80% of AIFRS cases [13].
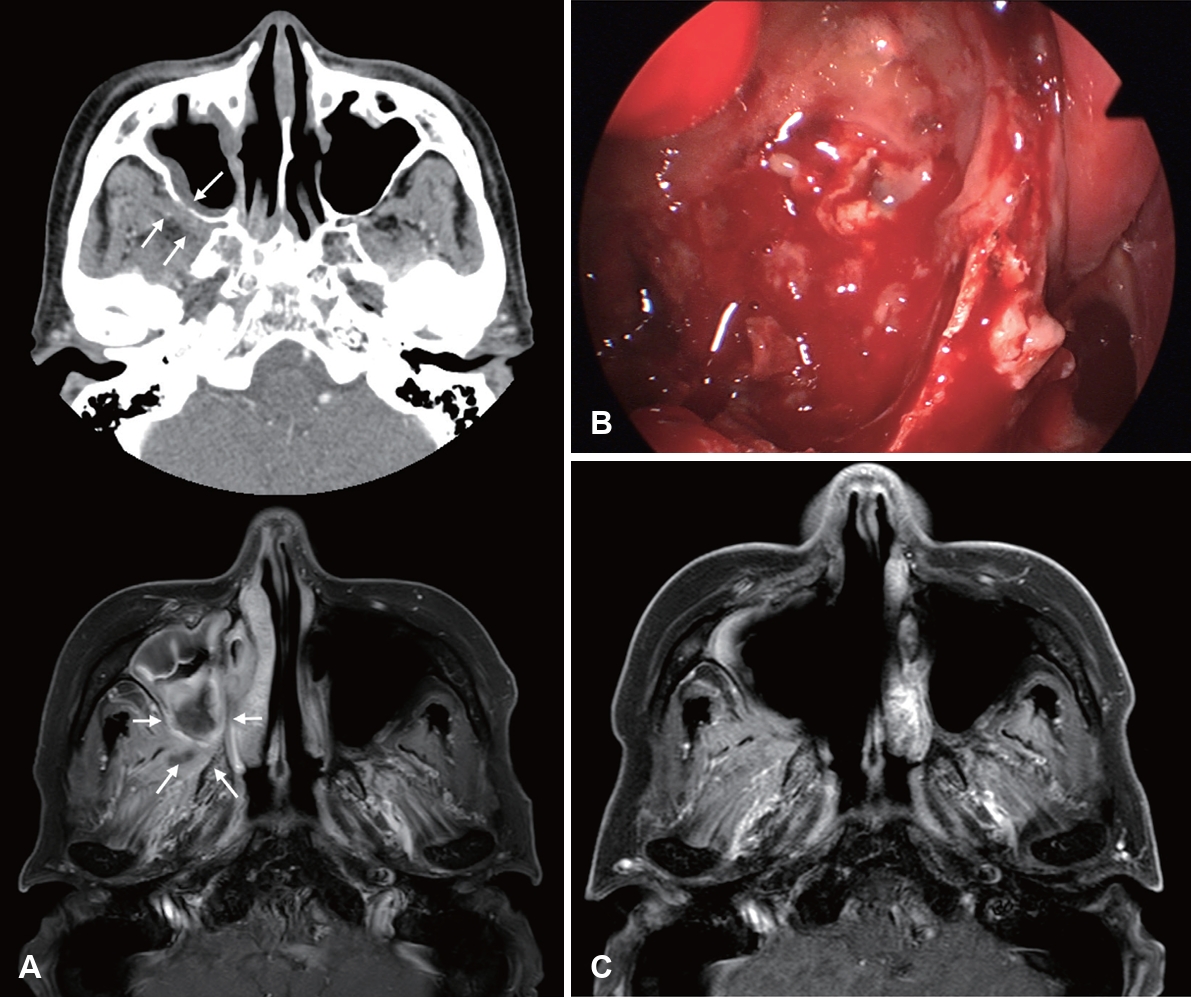
Gadolinium-enhanced MRI (Gd-MRI) has emerged as a superior modality for evaluating IFRS. The ŌĆ£black turbinate signŌĆØ of sinonasal tissue lacking contrast enhancement on MRI was first described to correspond to devitalized mucosa from angioinvasive hyphae [54]. However, it should be noted that nonenhancing portions of the turbinate are observed in 30% of patients without IFRS, especially in the posterior portion of inferior turbinates. Non-enhancing turbinates in immunocompetent patients retain peripheral (likely normal) mucosa enhancement and thin septa, which are key features distinguishing pathologic black turbinates from infiltrative nonenhancing lesions (IFRS), which exhibit infiltrative non-enhancement extending to adjacent structures without a smooth, thin enhancing margin [55]. MRI had a higher sensitivity than CT for diagnosing AIFRS with similar specificity, PPV, NPV, and accuracy, and LoCE on MRI showed 76.5% agreement with endoscopic mucosal findings. Therefore, MRI is more sensitive than CT in detecting early changes of AIFRS and is an appropriate initial diagnostic method when AIFRS is suspected, given suspicious endoscopic findings [52]. In 2017, the American College of Radiology Appropriateness Criteria recommended that an MRI of the face and sinuses, including orbit and brain, is the study of choice for evaluating patients with suspected IFRS. CT may be a valuable complement to MRI for surgical planning [56].
Furthermore, Lagos, et al. [41] reported that LoCE (75% sensitivity, 84% specificity, 50% PPV, and 94% NPV), extrasinonasal extension (60% sensitivity, 89% specificity, 60% PPV, and 89% NPV), and orbit compromise (50% sensitivity, 95% specificity, 75% PPV, and 86% NPV) on MRI were significantly associated with AIFRS. Kim, et al. [13] highlighted the significance of the loss of contrast enhancement (LoCE) on MRI as a characteristic finding of AIFRS. LoCE reflects tissue ischemia secondary to angiocentric invasion by fungal organisms. MRI is particularly valuable for the early detection of AIFRS, evaluation of extrasinonasal extension, assessment of intracranial and intraorbital involvement, distinguishing between viable and necrotic tissues, guiding the extent of surgical debridement, and monitoring treatment response (Fig. 2) [13]. Furthermore, LoCE at the skull base was reported as an independent poor prognostic factor (hazard ratio [HR]= 35.846, p=0.004) in patients with extrasinonasal IFRS, possibly because extensive necrotic lesions at the skull base cannot be removed entirely. A rather extensive resection may lead to serious morbidity, such as internal carotid artery injury, untreated cerebrospinal fluid leakage, meningitis, or brain damage [14].
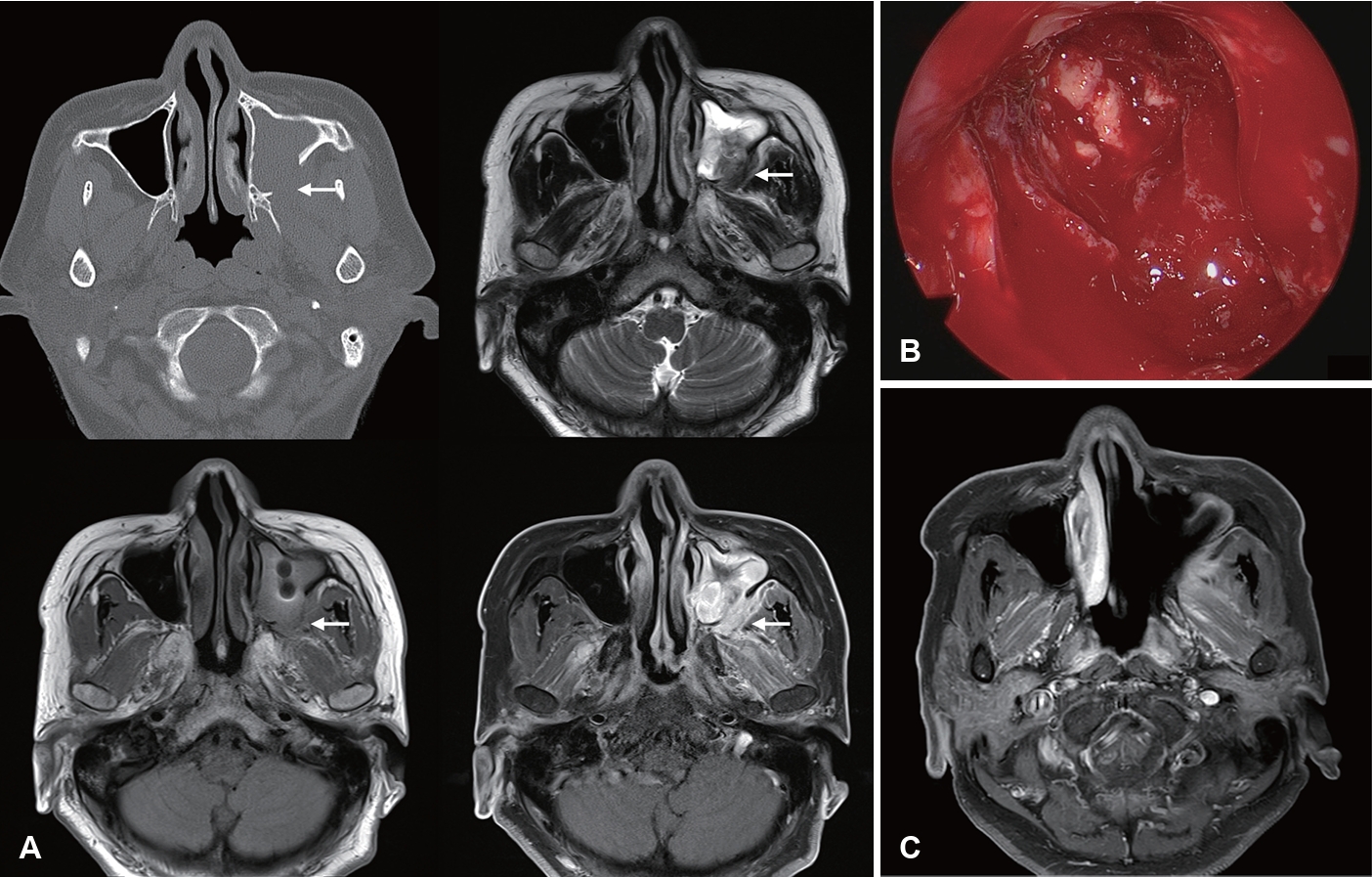
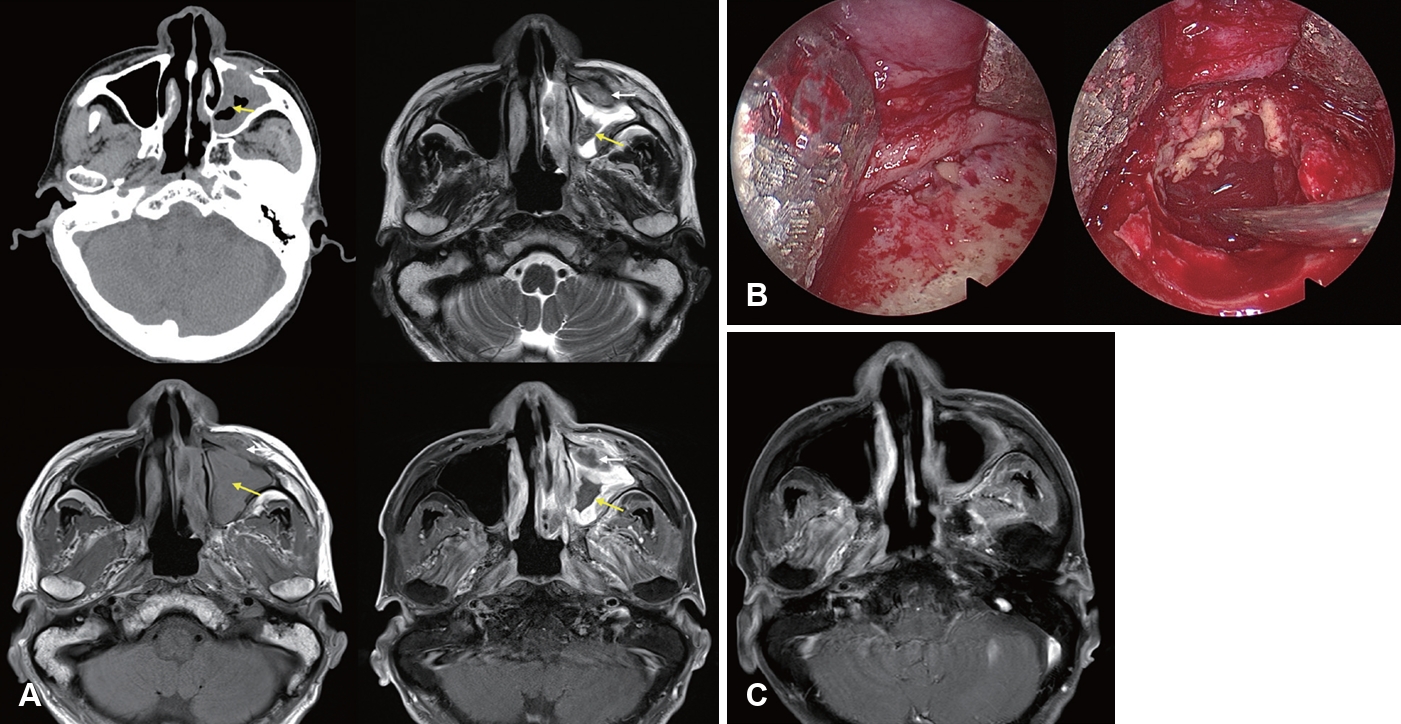
Image findings of CIFRS and CGIFRS are distinguished from those of AIFRS. CIFRS showed diffuse infiltrative patterns, whereas CGIFRS showed mass-forming patterns. The infiltrative pattern of CIFRS is characterized by a more extensive combined inflammation without focal mass formation (Fig. 3) [17]. The mass-forming pattern of CGIFRS is challenging to differentiate from malignancy (Fig. 4) [57,58]. On MRI, the T2 signal intensity of lesions of CIFRS was mainly intermediate (25%) to high (66%), whereas 50% of lesions of CGIFRS had low T2 signal intensity. Low T2 signal intensity is known to be associated with the presence of paramagnetic elements, such as iron and magnesium, or with hyphae [58,59]. Differences in inflammatory processes between CIFRS and CGIFRS may lead to differences in their radiologic features [17]. Other imaging features of CIFRS and CGIFRS are both bony sclerosis and bony erosion, with more than half showing tissue necrosis [17,60]. Sclerotic changes in bone are characteristic of a chronic course of sinusitis or underlying combined chronic sinusitis. In contrast, bony erosion and tissue necrosis may be indicators of the invasiveness of sinusitis [58,60]. Although these imaging findings may be important, they are not observed in all patients with CIFRS and CGIFRS and are therefore not diagnostic [17].
Histopathology and culture
Definite diagnosis of IFRS requires histopathological confirmation of fungal invasion into the sinonasal mucosa, submucosa, blood vessels, or bone. Bedside biopsy offers a low-cost tissue sampling method with the highest yield when endoscopic abnormalities are visible following initial endoscopic examination and imaging in suspected AIFRS cases. Patients with coagulopathy, thrombocytopenia, and medical comorbidities common in AIFRS require careful biopsy. Limited tissue collection and sampling bias restrict diagnostic utility in the absence of obvious findings [5]. A review found insufficient evidence supporting high-fidelity diagnosis of AIFRS by bedside biopsy and recommended comprehensive sinonasal evaluation and targeted biopsy in the operating room in clinically suspicious cases [61].
H&E staining remains the gold standard for permanent sections in IFRS diagnosis, and adjunct stains such as Gomori methenamine silver and Periodic Acid-Schiff are used to visualize fungal elements [21,23,62,63]. Key pathologic features of IFRS according to classification are that AIFRS shows fungal invasion of blood vessels with thrombosis and tissue necrosis, CIFRS shows dense accumulation of fungal hyphae with tissue and vascular invasion, and CGIFRS shows noncaseating granulomas with giant cells, vasculitis, and sparse fungal hyphae [1,2]. Frozen sections biopsy from the middle turbinate show variable sensitivity (74%-84%) [64,65], while targeted biopsies of discolored mucosa achieve sensitivity up to 91% but lower specificity (73%) [66]. The presence of hyphae doesnŌĆÖt confirm the invasive disease and random biopsies may yield false negatives. When noticeable mucosal changes exist, direct operative intervention for sampling and debridement may be preferable. Despite limitations, bedside biopsy can support treatment decisions when feasible.
Fungal culture of tissue specimens helps identify the causative organism, although the yield can be low [23,25,37,40,67,68]. Intraoperative tissue or aspirates maximize yield [69]. Culture sensitivity ranges from 36%-90% (typically 51.6%-67%), with specificity of 40%-85.7% [15,23,66-68,70]. Organism identification reduces mortality by guiding antifungal choice [4,24,70,71]. Limitations of the culture include low sensitivity for mucormycetes and non-Aspergillus molds/non-mucormycete molds [67,68,70], and a minimum 5-day turnaround time [66,67].
Direct microscopy of tissue specimens using potassium hydroxide (10% KOH, typically with fluorescence microscopy) is commonly performed in conjunction with fungal cultures, providing rapid fungal detection similar to intraoperative examination. Sensitivity ranges from 28.6%-60%, with specificity of 33.3%-100% [23,25,67]. The major limitation is an inability to identify specific pathogens, as molds in tissue lack species-identifying characteristics [72]. Despite this drawback, histopathology, fungal culture, and antifungal susceptibility testing are recommended for AIFRS cases, as they can detect infections missed by other assays [67].
Broad-range polymerase chain reaction (PCR) efficiently diagnoses AIFRS and identifies fungal species. A retrospective review showed a tissue PCR sensitivity of 85% compared to culture alone at 67.5%. A combined PCR-positive culture achieved 90% sensitivity and 78.5% specificity. PCR provides faster species-level identification and may detect polyfungal infections [67]. Combining PCR with mycologic culture is recommended to increase sensitivity and reduce turnaround time.
Prognostic associations with specific organisms are conflicting. Some studies reported that Aspergillus species [23] or so-called ŌĆ£atypicalŌĆØ (non-Aspergillus species/non-Mucormycetes) fungi [21] are associated with a poor prognosis. Conversely, other studies have demonstrated poorer outcomes with mucormycosis [7,24]. However, in our institutional experience, we have observed no significant difference in mortality rates based on causative fungal organisms (Mucormycetes vs. Aspergillus) [13,14].
For the evaluation of suspected IFRS, it is recommended to conduct histopathology cultures, direct tissue examination, antifungal susceptibility testing, and/or PCR analysis.
Treatment
Management of IFRS requires a multidisciplinary approach involving otorhinolaryngologists, infectious disease specialists, and other relevant specialties. Correction of underlying conditions, extensive surgical debridement, and systemic antifungal therapy are the three pillars of treatment.
Surgical management
Surgical debridement is a cornerstone of IFRS treatment, involving debridement of infected tissue until healthy, bleeding tissue is visualized [73]. Although early surgical intervention shows mixed evidence, most studies reported improved survival with early treatment. Optimal timing remains unclear, with cutoffs ranging from 4 to 16 days [49,73-76]. In patients with AIFRS, there is no evidence to guide the distinction between emergent surgical treatment (i.e., as soon as possible) and urgent surgical treatment (i.e., within 24 hours).
Complete surgical resection improves survival vs. incomplete resection [13,22,38,65]. However, guidelines for resection extent are lacking [22,32,64,77-79]. Surgical approach comparisons show conflicting results between open, endoscopic, and combined methods [6,12,74,80]. Appropriate surgical approaches can be chosen depending on the extent of the disease and the patientŌĆÖs general condition.
Our institution has established the principles for surgical management of IFRS based on evidence that postoperative extrasinonasal LoCE is significantly associated with mortality [13,14]. First, the extent of debridement is determined by intraoperative findings and preoperative imaging, particularly areas with LoCE on MRI. If necrotic tissue is identified during surgery and LoCE lesions found on preoperative Gd-MRI are in resectable locations without serious complications, the lesions are completely removed. CE lesions on preoperative Gd-MRI are not strictly removed and can be successfully treated with antifungal therapy, especially if located in the orbit or brain. Multiple debridement may be necessary based on disease progression. Endoscopic endonasal approaches are preferred when feasible. An open or combined approach may be required depending on the disease location, such as the anterior or inferior wall of the maxilla and hard palate. Orbital exenteration may be needed for extensive orbital involvement in patients with poor response to antifungal therapy. Urgent surgery is recommended upon diagnosis, especially for AIFRS. This strategy could reduce the IFRS mortality rate from 23.8% between 2003 and 2013 to 5.7% after 2013.
Antifungal therapy
Systemic antifungal therapy is essential and should be initiated empirically once IFRS is suspected. For mucormycosis, amphotericin B (preferably liposomal formulation) is the first-line agent. Posaconazole or isavuconazole may be used as step-down therapy. Voriconazole is the first-line agent for aspergillosis, with alternatives including isavuconazole, posaconazole, or amphotericin B. Therapy generally lasts for more than 3 months.
Correction of underlying condition
Diabetes is frequently reported in IFRS patients. Some studies report worse outcomes due to elevated glucose facilitating tissue invasion and impaired immunity [12,81]. Disease-specific mortality has been reported at 40%-76% in diabetic patients vs. 18%-47% in non-diabetic patients [24,82]. Conversely, other studies show no significant association with mortality [3,12,31], or even improved survival, possibly from reversible immunosuppression [50,83]. In our institutional experience, strict glycemic control (below 200 mg/dL) was associated with better survival in diabetic patients [13].
Neutropenia has traditionally been considered a risk factor for the development of AIFRS; however, it does not appear to be important in predicting survival outcomes in patients with AIFRS [3,13,14,21,84,85]. Conversely, restoration of neutrophil count with granulocyte transfusions and granulocyte colony-stimulating factor administration in patients with neutropenia appears to be an important prognostic factor [31,33,82]. In addition, remission of hematological diseases at the time of diagnosis is significantly associated with better AIFRS-specific survival [13].
Sinus fungus ball and progression to IFRS
Sinus fungus ball (SFB) can potentially progress to IFRS in elderly and immunocompromised patients [15]. SFB is typically considered a non-invasive form of fungal sinusitis characterized by the presence of fungal elements in the sinus cavity without tissue invasion. The authors reported 10 cases of concurrent SFB and IFRS, representing 1.6% of their SFB cases. Patients with combined SFB and IFRS were older (median age 70.5 years vs. 63 years for SFB alone), had higher rates of DM (50% vs. 27.6% for SFB alone), and more frequently had sphenoid sinus involvement (40% vs. 10.7% for SFB alone). This finding suggests that in certain high-risk populations, particularly elderly patients with diabetes or immunocompromising conditions, SFB may not always remain benign and should be monitored closely, with early surgical intervention considered.
Conclusion
IFRS remains a challenging clinical entity with significant morbidity and mortality. Early diagnosis, enabled by a high index of suspicion in at-risk patients and appropriate imaging studies, is crucial. Gd-MRI has emerged as a valuable tool for diagnosis, surgical planning, and prognostication, with LoCE serving as an essential marker of tissue necrosis and disease extent.
The management of IFRS requires a multidisciplinary approach, combining aggressive surgical debridement, appropriate antifungal therapy, and correction of underlying immunocompromising conditions. The potential for progression from SFB to IFRS in elderly and immunocompromised patients highlights the importance of vigilance and early intervention in high-risk populations.
Further research is needed to improve diagnostic methods, develop more effective antifungal therapies, and establish standardized management protocols to enhance outcomes in this challenging disease.